6.4: Enzyme Inhibition
- Last updated
- Save as PDF
- Page ID
- 102267
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Search Fundamentals of Biochemistry
Learning Goals (ChatGPT o1, 1/30/25)
-
Understanding Irreversible Covalent Inhibition:
- Explain how irreversible inhibitors modify key amino acid side chains (e.g., cysteine modification by iodoacetamide) to permanently inactivate an enzyme.
- Recognize the difference between enzyme denaturation (via extremes of pH and temperature) and irreversible covalent modification.
-
Mechanisms of Reversible Enzyme Inhibition:
- Define competitive inhibition and describe how substrate and inhibitor bind mutually exclusively to the active site.
- Interpret the competitive inhibition kinetic equation, v0=VMS/(KM(1+I/Kis)+S), and explain how this alters the apparent KM aoo without affecting VM.
-
Graphical Analysis of Competitive Inhibition:
- Interpret Lineweaver-Burk plots for competitive inhibition, identifying that the y-intercept (1/VM) remains constant while the x-intercept (–1/KM) shifts as inhibitor concentration increases.
- Explain how plotting v0 vs. log [S] can reveal the sigmoidal shifts associated with competitive inhibition.
-
Exploring Uncompetitive Inhibition:
- Describe uncompetitive inhibition where the inhibitor binds only to the enzyme–substrate (ES) complex, leading to a decrease in both VM and KM.
- Understand that Lineweaver-Burk plots for uncompetitive inhibition display parallel lines due to proportional decreases in both VM and KM.
-
Noncompetitive and Mixed Inhibition:
- Distinguish between noncompetitive inhibition (where the inhibitor binds to both free enzyme and ES with equal affinity) and mixed inhibition (with differing affinities).
- Interpret kinetic expressions for noncompetitive/mixed inhibition and explain how they affect VM and KM (e.g., KM remains unchanged in pure noncompetitive inhibition, while both parameters change in mixed inhibition).
- Analyze interactive graphs and Lineweaver-Burk plots to visualize these effects.
-
Product Inhibition and Competing Substrate Effects:
- Explain how product inhibition occurs when the reaction product, due to its structural similarity to the substrate, binds to the enzyme and decreases the overall reaction rate.
- Understand the concept of specificity constants (kcat/KM) and how competing substrates can effectively act as inhibitors.
-
In Vivo vs. In Vitro Inhibition:
- Contrast the experimental conditions for enzyme inhibition studies in vitro (fixed enzyme, varying substrate) versus in vivo (dynamic flux through metabolic pathways).
- Interpret how metabolic flux and substrate accumulation can influence the effectiveness of competitive versus uncompetitive inhibitors in a cellular context.
-
Environmental Effects on Enzyme Activity:
- Describe how temperature and pH changes affect enzyme activity, including the role of pH in altering the protonation states of key catalytic residues.
- Predict how variations in temperature or pH might lead to reversible changes in enzyme kinetics or irreversible enzyme denaturation.
By achieving these learning goals, students will gain a thorough understanding of both the mechanistic basis of enzyme inhibition and the practical techniques used to measure and analyze inhibitor effects. This knowledge forms a critical foundation for exploring enzyme regulation in metabolic pathways and designing effective pharmaceutical inhibitors.
Irreversible Covalent Inhibition
Given what you already know about protein structure, it should be easy to determine how to inhibit an enzyme. Since structure mediates function, anything that would significantly alter an enzyme's structure would inhibit the enzyme's activity. Hence, extremes of pH and high temperature, all of which can denature the enzyme, would irreversibly inhibit the enzyme unless it could refold properly. Alternatively, we could add a small molecule, which interacts noncovalently with the enzyme to either change its conformation or directly prevent substrate binding. Finally, we could covalently modify certain side chains, which, if they are essential to enzymatic activity, would irreversibly inhibit the enzyme.
We discussed previously the types of reagents that would chemically modify specific side chains that might be critical for enzymatic activity. For example, iodoacetamide might abolish enzyme activity if a cysteine side chain is required for activity. However, these reagents will usually modify several side chains. Determining which is critical for the binding or catalytic conversion of the substrate can be difficult. One way would be to protect the active site with saturating concentrations of a ligand that binds reversibly at the active site. Then, the chemical modification can be performed at varying reaction times. The critical side chain would be protected from chemical modification, with the extent of protection depending on the KD, the concentration of the protecting ligand, and the length of the reaction.
The rest of the chapter will deal with reversible, noncovalent inhibition
Competitive Inhibition
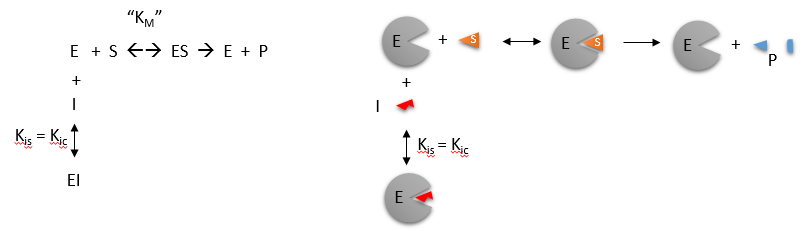
Reversible Competitive inhibition occurs when substrate (S) and inhibitor (I) both bind to the same site on the enzyme. In effect, they compete for the active site and bind in a mutually exclusive fashion. This is illustrated in the chemical equations and molecular cartoons shown in Figure \(\PageIndex{1}\).
\begin{equation}
v_0=\frac{V_M S}{K_M\left(1+\frac{I}{K is}\right)+S}
\end{equation}
There is another type of inhibition that would give the same kinetic data. If S and I were bound to different sites, and S was bound to E and produced a conformational change in E such that I could not bind (and vice versa), then the binding of S and I would be mutually exclusive. This is called allosteric competitive inhibition. Inhibition studies are usually done at several fixed and non-saturating concentrations of I and varying S concentrations.
The key kinetic parameters to understand are VM and KM. Let us assume for ease of equation derivation that I binds reversibly and with rapid equilibrium to E, with a dissociation constant KIS. The "s" in the subscript "is" indicates that the slope of the 1/v vs. 1/S Lineweaver-Burk plot changes while the y-intercept stays constant. KIS is also named KIC, where the subscript "c" stands for competitive inhibition constant.
A look at the top mechanism shows that even in the presence of I, as S increases to infinity, all E is converted to ES. That is, there is no free E to which I could bind. Now, remember that VM= kcatE0. Under these conditions, ES = E0; hence v = VM. VM is not changed. However, the apparent KM, KMapp, will change. We can use LaChatelier's principle to understand this. If I binds to E alone and not ES, it will shift the equilibrium of E + S → ES to the left. This would increase the KMapp (i.e., it would appear that the affinity of E and S has decreased). The double reciprocal plot (Lineweaver-Burk plot) offers a great way to visualize the inhibition as shown in Figure \(\PageIndex{2}\).
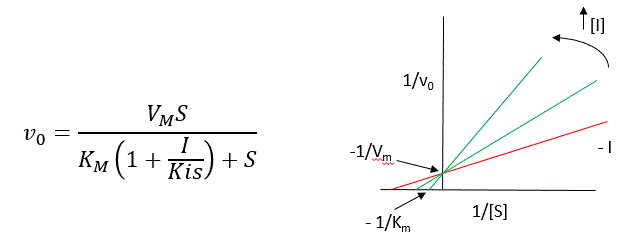
In the presence of I, VM does not change, but KM appears to increase. Therefore, 1/KM, the x-intercept on the plot, will get smaller and closer to 0. Therefore, the plots will consist of a series of lines, with the same y-intercept (1/VM), and the x-intercepts (-1/KM) closer and closer to 0 as I increases. These intersecting plots are the hallmarks of competitive inhibition.
Here is an interactive graph showing v0 vs [S] for competitive inhibition with Vm and Km both set to 100. Change the sliders for [I] and Kis and see the effect on the graph.
Here is the interactive graphs showing 1/v0 vs 1/[S] for competitive inhibition, with Vm and Km both set to 10.
Note that in the first three inhibition models discussed in this section, the Lineweaver-Burk plots are linear in the presence and absence of an inhibitor. This suggests that plots of v vs. S in each case would be hyperbolic and conform to the usual form of the Michaelis-Menten equation, each with potentially different apparent VM and KM values.
An equation for v0 in the presence of a competitive inhibitor is shown in the above figure. The only change compared to the equation for the initial velocity in the absence of the inhibitor is that the KM term is multiplied by the factor 1+I/Kis. Hence KMapp = KM(1+I/Kis). This shows that the apparent KM does increase as we predicted. KIS is the inhibitor dissociation constant. The apparent KM changes with increasing I, which affects the slope of the double reciprocal plot.
If the data were plotted as v0 vs. log S, the plots would be sigmoidal, as we saw for plots of ML vs. log L in Chapter 5B. In the case of a competitive inhibitor, the plot of v0 vs log S in the presence of different fixed concentrations of inhibitor would consist of a series of sigmoidal curves, each with the same VM, but with different apparent KM values (where KMapp = KM(1+I/Kis), progressively shifted to the right. Enzyme kinetic data is rarely plotted this way. These plots are mostly used for simple binding data for the M + L ↔ ML equilibrium in the presence of different inhibitor concentrations.
Reconsider our discussion of the simple binding equilibrium, M + L ↔ ML. For fractional saturation Y vs a log L graphs, we considered three examples:
- L = 0.01 KD (i.e., L << KD), which implies that KD = 100L. Then Y = L/[KD+L] = L/[100L + L] ≈1/100. This implies that irrespective of the actual [L], if L = 0.01 KD, then Y ≈0.01.
- L = 100 KD (i.e,. L >> KD), which implies that KD = L/100. Then Y = L/[KD+L] = L/[(L/100) + L] = 100L/101L ≈ 1. This implies that irrespective of the actual [L], if L = 100 Kd, then Y ≈1.
- L = KD, then Y = 0.5
These scenarios show that if L varies over four orders of magnitude (0.01KD < KD < 100KD), or, in log terms, from
-2 + log KD < log KD< 2 + log KD), irrespective of the magnitude of the KD, that Y varies from approximately 0 - 1.
In other words, Y varies from 0-1 when L varies from log KD by +2. Hence, plots of Y vs log L for a series of binding reactions of increasingly higher KD (lower affinity) would reveal a series of identical sigmoidal curves shifted progressively to the right, as shown below in Figure \(\PageIndex{3}\).

The same would be true of v0 vs. S in the presence of different concentrations of a competitive inhibitor, for initial flux, Jo vs ligand outside, in the presence of a competitive inhibitor, or ML vs. L (or Y vs L) in the presence of a competitive inhibitor.
In many ways, plots of v0 vs. lnS are easier to visually interpret than plots of v0 vs. S . As noted for simple binding plots, textbook illustrations of hyperbolas are often misdrawn, showing curves that level off too quickly as a function of [S] as compared to plots of v0 vs lnS, in which it is easy to see if saturation has been achieved. In addition, as the curves above show, multiple complete plots of v0 vs lnS at varying fixed inhibitor concentrations or for variant enzyme forms (different isoforms, site-specific mutants) over a broad range of lnS can be made, which facilitates comparisons of the experimental kinetics under these different conditions. This is especially true if Km values differ widely.
Now that you are more familiar with binding and enzyme kinetics curves, in the presence and absence of inhibitors, you should be able to apply the above analysis to inhibition curves where the binding or the initial velocity is plotted at varying competitive inhibitor concentrations at different fixed nonsaturating concentrations of ligand or substrate. Consider the activity of an enzyme. Let's say that at some reasonable substrate concentration (not infinite), the enzyme is approximately 100% active. If a competitive inhibitor is added, the activity of the enzyme decreases until at saturating (infinite) I, no activity would remain. Graphs showing this are shown below in Figure \(\PageIndex{4}\).
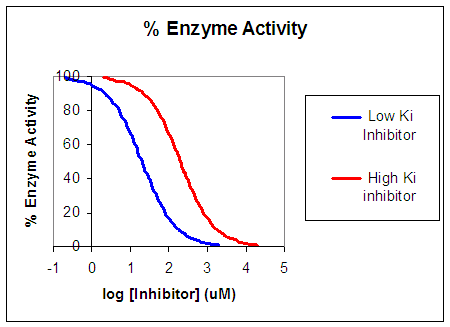
Progress Curves for Competitive Inhibition
In the previous section, we explored how important progress curve (Product vs time) analyses are in understanding both uncatalyzed and enzyme-catalyzed reactions. We are aware of no textbooks that cover progress curves for enzyme inhibition. Yet progress curves are what most investigators record and analyze to determine initial rates v0 and to calculate VM, KM, and inhibition constants, as described above. We will use Vcell to produce progress curves for reversibly inhibited enzyme-catalyzed reactions.
 MODEL
MODEL
Competitive Inhibition with constant [I]:
No inhibition (left) and competitive inhibition (right)
Initial conditions for no inhibition
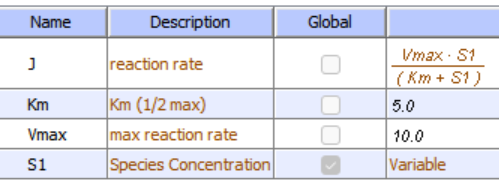
Initial conditions for competitive inhibition
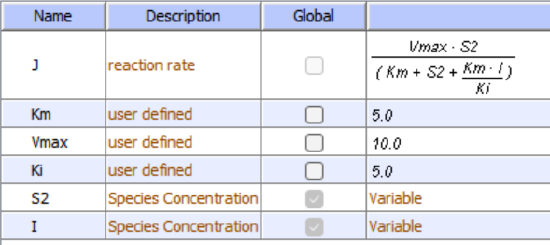
I is fixed for each simulation (as it is not converted to a product) but can be changed in the simulation below.
Select Load [model name] below
Select Start to begin the simulation.
Interactive Element
Select Plot to change Y axis min/max, then Reset and Play | Select Slider to change which constants are displayed. For this model, select Vm, Km, Ki and I | Select About for software information.
Move the sliders to change the constants and see changes in the displayed graph in real-time.
Time course model made using Virtual Cell (Vcell), The Center for Cell Analysis & Modeling, at UConn Health. Funded by NIH/NIGMS (R24 GM137787); Web simulation software (miniSidewinder) from Bartholomew Jardine and Herbert M. Sauro, University of Washington. Funded by NIH/NIGMS (RO1-GM123032-04)
The graphs from your initial run show the concentrations of S, P, and I as a function of time for just the initial conditions shown above. In typical initial rate analyses of competitive inhibition, at least three sets of reactions are run with the same varying substrate concentrations and different fixed inhibitor concentrations. In the analyses above, [I] is fixed at 5 uM.
Conduct a series of runs at different values of I. Vary the KI, the dissociation constant for the EI complex, as follows:
- I << KI, the dissociation constant for the EI complex
- I >> KI, the dissociation constant for the EI complex. Then, download the data and determine the initial rate for each of the initial conditions.
Figure \(\PageIndex{5}\) shows an interactive iCn3D model of human low molecular weight phosphotyrosyl phosphatase bound to a competitive inhibitor (5PNT)
Figure \(\PageIndex{5}\): Human low molecular weight phosphotyrosyl phosphatase bound to a competitive inhibitor (5PNT). (Copyright; author via source).
Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...XsEacG2tixDDi9
The competitive inhibitor, the deprotonated form of 2-(N-morpholino)-ethanesulfonic acid (MES), is actually the conjugate base of the weak acid (pKa = 6.15) of a commonly used component of a buffered solution. It is shown in color sticks with the negatively charged sulfonate at the bottom of the active site pocket. The amino acids comprising the active site binding pocket are shown as color sticks underneath the transparent colored surface of the binding pocket. The normal substrates for the enzyme are proteins phosphorylated on tyrosine side chains, so the sulfonate mimics the negatively charged phosphate group of the phosphoprotein target.
Two special cases of competition inhibition
Product Inhibition
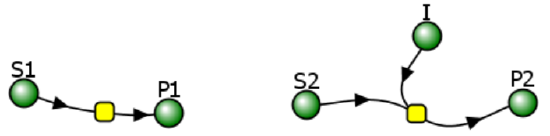
Let's look at an enzyme that converts reactant S to product P. Since P arises from S, they may have structural similarities. For example, what if GTP is the reactant and GDP the product? If so, then P might also bind in the active site and inhibit the conversion of S to P. This is called product inhibition. It probably occurs in most enzymes, and when it does occur, it will start bending downward at the beginning of the progress curve for P formation. If the product binds very tightly, it might cause a significant underestimation of the initial velocity (v0) or flux (J0) of the enzyme. Let's use Vcell to explore product inhibition. The model will explore two reactions:
- E + R ↔ ER → E + Q (no product inhibition)
- E + S ↔ ES → E + P (with product inhibition)
The chemical equation above does not explicitly show the product P binding the enzyme to form an EP complex. An actual reaction diagram showing the inhibition of an enzyme by an inhibitor I and by the product P is shown in Figure \(\PageIndex{6}\) below.
Figure \(\PageIndex{6}\): reaction diagram showing inhibition of an enzyme by an inhibitor I and by the product P
Vcell uses much simpler diagrams since it is most often used for modeling whole pathways or even entire cells. In the simpler Vcell reaction diagrams, the inhibitor is typically not shown since the inhibition is built into the equation for the enzyme, represented by the node or yellow square in the figure above.
Let's now explore product inhibition in Vcell. In the reaction without product inhibition, R and Q are the reactant and product, respectively. S and P are used for the reaction with product P inhibition.
 MODEL
MODEL
Irreversible MM Kinetics - Without (left rx 1) and With (right, rx 2) Product Inhibition
Initial Conditions: No product inhibition
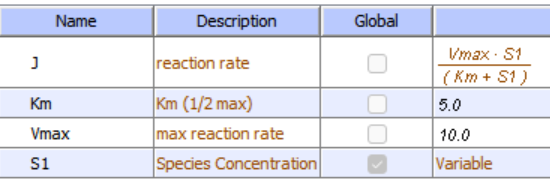
Initial Conditions: With product inhibition
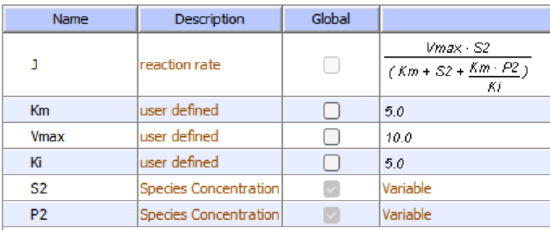
Select Load [model name] below
Select Start to begin the simulation.
Interactive Element
Select Plot to change Y axis min/max, then Reset and Play | Select Slider to change which constants are displayed | Select About for software information.
Move the sliders to change the constants and see changes in the displayed graph in real-time.
Time course model made using Virtual Cell (Vcell), The Center for Cell Analysis & Modeling, at UConn Health. Funded by NIH/NIGMS (R24 GM137787); Web simulation software (miniSidewinder) from Bartholomew Jardine and Herbert M. Sauro, University of Washington. Funded by NIH/NIGMS (RO1-GM123032-04)
Inhibition by a competing substrate - the specificity constant
In the previous chapter, the specificity constant was defined as kcat/KM, which we also described as the second-order rate constant associated with the bimolecular reaction of E and S when S << KM. It also describes how good an enzyme is at differentiating between different substrates. If an enzyme encounters two different substrates, one can be considered a competitive inhibitor of the other. The following equation gives the ratio of initial velocities for two competing substrates at the same concentration equal to the ratio of their kcat/KM values.
\begin{equation}
\frac{\mathrm{v}_{\mathrm{A}}}{\mathrm{v}_{\mathrm{B}}}=\frac{\frac{\mathrm{k}_{\mathrm{catA}}}{\mathrm{K}_{\mathrm{A}}} \mathrm{A}}{\frac{\mathrm{k}_{\mathrm{cat} \mathrm{B}}}{\mathrm{K}_{\mathrm{B}}} \mathrm{B}}
\end{equation}
A derivation of the specificity constant for an enzyme with competing substrates
Here it is!
- Derivation
-
\begin{equation}
\mathrm{v}_{\mathrm{A}}=\frac{\mathrm{V}_{\mathrm{A}} \mathrm{A}}{\mathrm{K}_{\mathrm{A}}\left(1+\frac{\mathrm{B}}{\mathrm{K}_{\mathrm{B}}}\right)+\mathrm{A}} \quad \mathrm{v}_{\mathrm{B}}=\frac{\mathrm{V}_{\mathrm{B}} \mathrm{B}}{\mathrm{K}_{\mathrm{B}}\left(1+\frac{\mathrm{A}}{\mathrm{K}_{\mathrm{A}}}\right)+\mathrm{B}}
\end{equation}\begin{equation}
\frac{\mathrm{v}_{\mathrm{A}}}{\mathrm{v}_{\mathrm{B}}}=\frac{\frac{\mathrm{V}_{\mathrm{A}} \mathrm{A}}{\mathrm{K}_{\mathrm{A}}\left(1+\frac{\mathrm{B}}{\mathrm{K}_{\mathrm{B}}}\right)+\mathrm{A}}}{\frac{\mathrm{V}_{\mathrm{B}} \mathrm{B}}{\mathrm{K}_{\mathrm{B}}\left(1+\frac{\mathrm{A}}{\mathrm{K}_{\mathrm{A}}}\right)+\mathrm{B}}}=\frac{\frac{\mathrm{V}_{\mathrm{A}} \mathrm{A}}{\mathrm{K}_{\mathrm{A}}+\frac{\mathrm{K}_{\mathrm{A}} \mathrm{B}}{\mathrm{K}_{\mathrm{B}}}+\mathrm{A}}}{\frac{\mathrm{V}_{\mathrm{B}} \mathrm{B}}{\mathrm{K}_{\mathrm{B}}+\frac{\mathrm{K}_{\mathrm{B}} \mathrm{A}}{\mathrm{K}_{\mathrm{A}}}+\mathrm{B}}}
\end{equation}Now, in the above equation:
multiple the top half of the right-hand expression by \begin{equation}
\frac{\frac{1}{K_A}}{\frac{1}{K_A}}
\end{equation} multiple the bottom half of the right-hand expression by \begin{equation}
\frac{\frac{1}{K_B}}{\frac{1}{K_B}}
\end{equation} replace VA with kcatAE0 and VB with kcatBE0This gives the following expression for vA/vB:
\begin{equation}
\frac{\mathrm{v}_{\mathrm{A}}}{\mathrm{v}_{\mathrm{B}}}=\frac{\frac{\mathrm{k}_{\mathrm{catA}}}{\mathrm{K}_{\mathrm{A}}} \mathrm{A}}{\frac{\mathrm{k}_{\mathrm{cat} \mathrm{B}}}{\mathrm{K}_{\mathrm{B}}} \mathrm{B}}
\end{equation}
Uncompetitive Inhibition
Reversible uncompetitive inhibition occurs when I binds only to ES and not free E. One can hypothesize that on binding S, a conformational change in E occurs, which presents a binding site for I. Inhibition occurs since ESI can not form the product. It is a dead-end complex that has only one fate: to return to ES. This is illustrated in the chemical equations and molecular cartoon shown in Figure \(\PageIndex{7}\).
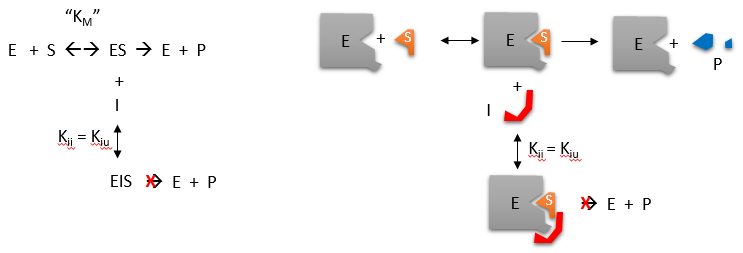
\begin{equation}
v_0=\frac{V_M S}{K_M+S\left(1+\frac{I}{K i i}\right)}=\frac{\left(\frac{V_M}{1+\frac{I}{K i i}}\right) S}{\left(\frac{K_M}{1+\frac{I}{K i i}}\right)+S}
\end{equation}
Let us assume that I binds reversibly to ES with a dissociation constant Kii. The second "i" in the subscript "ii" indicates that the intercept of the 1/v vs 1/S Lineweaver-Burk plot changes while the slope stays constant. Kii is also named Kiu, where the subscript "u" stands for the uncompetitive inhibition constant.
A look at the top mechanism shows that in the presence of I, as S increases to infinity, not all of E is converted to ES. There is a finite amount of ESI, even at an infinite S. Now remember that Vm = kcatE0 if and only if all E is in the form ES. Under these conditions, the apparent Vm, Vmapp is less than the real Vm without the inhibitor. In addition, the apparent Km, Kmapp, will change. We can use LaChatelier's principle to understand this. If I binds to ES alone, and not E, it will shift the equilibrium of E + S ↔ ES to the right, which would have the effect of decreasing the Kmapp (i.e., it would appear that the affinity of E and S has increased). The double reciprocal plot (Lineweaver-Burk plot) offers a great way to visualize the inhibition. In the presence of I, both Vm and Km decrease. Therefore, -1/Km, the x-intercept on the plot, will get more negative, and 1/Vm will get more positive. It turns out that they change to the same extent. Therefore, the plots will consist of a series of parallel lines, which is the hallmark of uncompetitive inhibition, as shown in Figure \(\PageIndex{8}\).

Here is an interactive graph showing v0 vs [S] for uncompetitive inhibition with Vm and Km both set to 100. Change the sliders for [I] and Kis and see the effect on the graph.
Here is an interactive graph showing uncompetitive inhibition with Vm and Km both set to 10. Change the sliders for [I] and Kii and see the effect on the graph
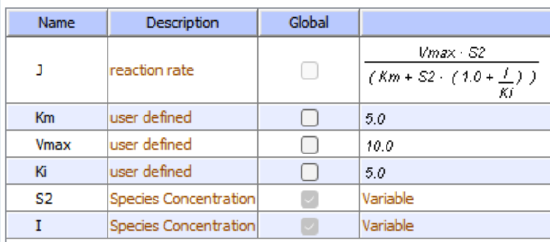
An equation, shown in the diagram above, can be derived to show the effect of the uncompetitive inhibitor on the velocity of the reaction. The only change is that the S term in the denominator is multiplied by the factor 1+I/Kii. We want to rearrange this equation to show how Km and Vm are affected by the inhibitor, not S. Rearranging the equation above shows that Kmapp = Km/(1+I/Kii) and Vmapp = Vm/(1+I/Kii). This shows that the apparent Km and Vm do decrease as we predicted. Kii is the inhibitor dissociation constant in which the inhibitor affects the intercept of the double reciprocal plot. Note that if I is zero, Km and Vm are unchanged.
Progress Curves: Uncompetitive Inhibition
Now, let's compare the progress curves for an enzyme-catalyzed reaction in the absence and presence of an uncompetitive inhibitor.
 MODEL
MODEL
No inhibition (left) and Uncompetitive Inhibition (right)
(Note the the Vcell reaction diagram is the same as for competitive inhibition. However, the mathematical equations differ as shown below.
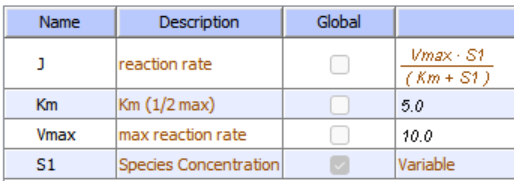
Initial values No Inhibition
Initial values With Uncompetitive Inhibitor
I is fixed for each simulation (as it is not converted to a product) but can be changed in the simulation below.
Select Load [model name] below
Select Start to begin the simulation.
Interactive Element
Select Plot to change Y axis min/max, then Reset and Play | Select Slider to change which constants are displayed | Select About for software information.
Move the sliders to change the constants and see changes in the displayed graph in real-time.
Time course model made using Virtual Cell (Vcell), The Center for Cell Analysis & Modeling, at UConn Health. Funded by NIH/NIGMS (R24 GM137787); Web simulation software (miniSidewinder) from Bartholomew Jardine and Herbert M. Sauro, University of Washington. Funded by NIH/NIGMS (RO1-GM123032-04)
Figure \(\PageIndex{9}\) shows an interactive iCn3D model of an uncompetitive inhibitor of the cysteine protease caspase-6 (4HVA)
Figure \(\PageIndex{9}\): Uncompetitive inhibitor of the cysteine protease caspase-6 (4HVA) . (Copyright; author via source).
Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...TVfKio7NxcpX28
The "substrate" in this model (cyan, transparent surface, with labels on V, E, and I) is a substrate analog, VEI-CHO, in which the tripeptide substrate VEI ends not in a free carboxyl or amide group but an aldehyde, which causes this "substrate" to become covalently attached to the enzyme and act as an inhibitor. The uncompetitive inhibitor (gray transparent surface) binds externally to the blue surface. Hence, it binds to the ES complex. Two active site residues, Cys 163, the active site nucleophile, and His 121, a catalytic acid/base, are shown in colored sticks and labeled.
Figure \(\PageIndex{10}\) below shows a Lineweaver-Burk plot showing 1/vo vs 1/[S], where the substrate is the divalent compound (VEID)2R110. Its N-terminus is capped with a benzyloxy (Z) group. R110 is a rhodamine-type fluorophore, which, on cleavage, gives a strong fluorescent signal that the benzyloxy group initially quenches in the uncleaved substrate.
Figure \(\PageIndex{10}\): Double-reciprocal Lineweaver-Burk plot of compound 3 with (VEID)2R110 substrate showing uncompetitive inhibition. Heise CE et al. (2012) Mechanistic and Structural Understanding of Uncompetitive Inhibitors of Caspase-6. PLoS ONE 7(12): e50864. https://doi.org/10.1371/journal.pone.0050864. Creative Commons Attribution License.
Noncompetitive and Mixed Inhibition
Reversible noncompetitive inhibition occurs when I binds to both E and ES. We will look at only the special case in which the dissociation constants of I for E and ES are the same. This is called noncompetitive inhibition. It is quite rare, as it would be difficult to imagine a large inhibitor that inhibits the turnover of a bound substrate and does not affect the binding of S to E. However, the covalent interaction of protons with both E and ES can lead to noncompetitive inhibition. In the more general case, the Kd's are different, and the inhibition is called mixed. Since inhibition occurs, we will hypothesize that ESI can not form the product. It is a dead-end complex with only one fate: to return to ES or EI. This is illustrated in the chemical equations and the molecular cartoon in Figure \(\PageIndex{11}\).
Let us assume for ease of equation derivation that I binds reversibly to E with a dissociation constant of Kis (as we denoted for competitive inhibition) and to ES with a dissociation constant Kii (as we noted for uncompetitive inhibition). Assume that for noncompetitive inhibition, Kis = Kii. A look at the top mechanism shows that in the presence of I, as S increases to infinity, not all of E is converted to ES. There is a finite amount of ESI, even at infinite S. Now remember that Vm = kcatE0 if and only if all E is in the form ES. Under these conditions, the apparent Vm, Vmapp, is less than the real Vm without an inhibitor. In contrast, the apparent Km, Kmapp, will not change since I binds to both E and ES with the same affinity and hence will not perturb that equilibrium, as deduced from LaChatelier's principle. The double reciprocal plot (Lineweaver-Burk plot) offers a great way to visualize the inhibition. In the presence of I, just Vm will decrease. Therefore, -1/Km, the x-intercept will stay the same, and 1/Vm will get more positive. Therefore, the plots will consist of a series of lines intersecting on the x-axis, the hallmark of noncompetitive inhibition. You should be able to figure out how the plots would appear if Kis is different from Kii (mixed inhibition).
\begin{equation}
v_0=\frac{V_M S}{K_M\left(1+\frac{I}{K i s}\right)+S\left(1+\frac{I}{K i i}\right)}
\end{equation}
An equation, shown in the diagram above, can be derived to show the effect of the noncompetitive inhibitor on the velocity of the reaction. Km is multiplied by 1+I/Kis, and S by 1+I/Kii in the denominator. We want to rearrange this equation to show how the inhibitor affects Km and Vm, not S. Rearranging the equation as shown above shows that Kmapp = Km(1+I/Kis)/(1+I/Kii) = Km when Kis=Kii, and Vmapp = Vm/(1+I/Kii). This shows that the Km is unchanged, and Vm decreases as we predicted. The plot shows a series of lines intersecting on the x-axis as shown in Figure \(\PageIndex{12}\). Both the slope and the y-intercept are changed, which are reflected in the names of the two dissociation constants, Kis and Kii. Note that if I is zero, Kmapp = Km and Vmapp = Vm. Sometimes, the Kis and Kii inhibition dissociation constants are called Kc and Ku (competitive and uncompetitive inhibition dissociation constants.
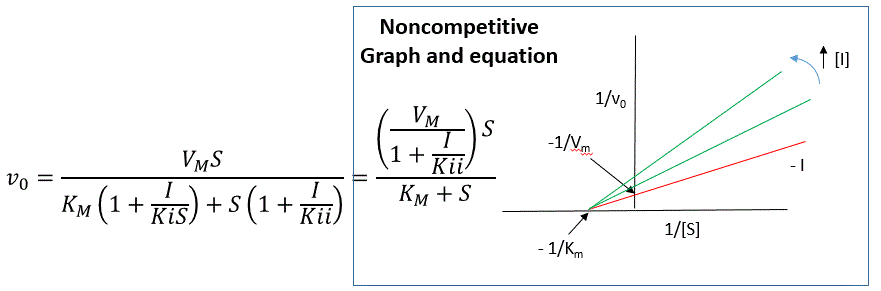
Move the sliders on this interactive graph below to show changes in Kis and Kii affect the position on the graph where the lines intersect. Try to change their values to move the intersections of the graphs from the left top quadrant to the x-axis to the left bottom quadrant.
Here is an interactive graph showing v0 vs [S] for mixed inhibition with Vm and Km both set to 100. Change the sliders for [I] and Kis and see the effect on the graph
Here is an interactive graph showing mixed inhibition with Vm and Km both set to 10. Change the sliders for [I] and Kii and see the effect on the graph
Progress Curves: Mixed/Noncompetitive Inhibition
Now let's compare the progress curves for an enzyme-catalyzed reaction in the absence and presence of a mixed inhibitor.
 MODEL
MODEL
No inhibition (left) and Uncompetitive Inhibition (right)

Note that the Vcell reaction diagram is the same as for competitive and uncompetive inhibition. It doesn't explicitly show that the mixed inhibitor binds to both free and substrate-bound enzymes. Those interactions are addressed in the mathematical equations for mixed inhibition.
Initial values No Inhibition

Initial values With Uncompetitive Inhibitor
I is fixed for each simulation (as it is not converted to a product) but can be changed in the simulation below.
Select Load [model name] below
Select Start to begin the simulation.
Interactive Element
Select Plot to change Y axis min/max, then Reset and Play | Select Slider to change which constants are displayed | Select About for software information.
Move the sliders to change the constants and see changes in the displayed graph in real-time.
Time course model made using Virtual Cell (Vcell), The Center for Cell Analysis & Modeling, at UConn Health. Funded by NIH/NIGMS (R24 GM137787); Web simulation software (miniSidewinder) from Bartholomew Jardine and Herbert M. Sauro, University of Washington. Funded by NIH/NIGMS (RO1-GM123032-04)
Mixed and noncompetitive inhibition (as shown by the mechanism above) differ from competitive and uncompetitive inhibition. The inhibitor binding is not simply a dead-end reaction in which the inhibitor can only dissociate in a single reverse step. In the above equilibrium, S can dissociate from ESI to form EI, so the system may not be at equilibrium. With dead-end steps, no flux of reactants occurs through the dead-end complex, so the equilibrium for the dead-end step is not perturbed.
Other mechanisms can commonly give mixed inhibition. For example, the product released in a ping pong mechanism (discussed in the next chapter) can give mixed inhibition. A ping pong reaction mechanism is shown and superficially explained in Figure \(\PageIndex{13}\).
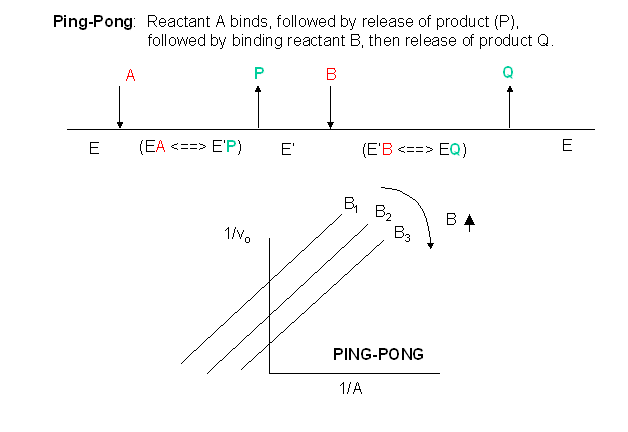
If P, acting as a product inhibitor, can bind to two different forms of the enzyme (E' and also E), it will act as a mixed inhibitor.
Figure \(\PageIndex{14}\) shows an interactive iCn3D model of a noncompetitive inhibitor of M. tuberculosis's class IIa fructose 1,6-bisphosphate aldolase (4LV4).
Figure \(\PageIndex{14}\): Noncompetitive inhibitor of M. tuberculosis's class IIa fructose 1,6-bisphosphate aldolase (4LV4). (Copyright; author via source).
Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...wZDtkvtwqnrVy7
The non-competitive inhibitor, 8-hydroxyquinoline carboxylic acid (HCA), is shown with a cyan surface. Active site side chains of the Zn2+- containing enzyme are shown as colored sticks and labeled.
The noncompetitive inhibitor does not enter into the pocket where the substrate (not shown in the model above) binds. The binding of the noncompetitive inhibitor causes a conformational change that moves the Zn-binding loop (Z-loop), which contains His 212, which coordinates the Zn2+ ion. Hence, the Zn-loop is part of the active site, and when it moves on binding of the inhibitor, the access to the empty pocket where the substrate binds is hindered. When the Zn2+ moves away from the active site, it can no longer engage in catalytic activity.
Enzyme Inhibition in Vivo
The pharmaceutical industry is devoted to finding drug molecules that affect biological processes. Typically, this means the development of small-molecule inhibitors for target proteins. Recent work has expanded to developing inhibitory RNA molecules that affect DNA transcription and mRNA translation. Using combinatorial synthetic techniques and computational modeling, developing small molecule inhibitors (especially competitive ones) that inhibit proteins in vitro using purified enzymes, substrates, and inhibitors in lab testing has gotten easier. Assuming that the inhibitor could pass through the membrane and accumulate to a sufficient concentration, would it have the same inhibitory properties in the cell as in the test tube? The answer turns out to be maybe. Remember that a cell is tightly packed with a multitude of other small molecules and macromolecules. In addition, the enzyme targeted for inhibition is most likely part of a pathway of enzymes that feed reactants into the enzyme and remove the product. Hence, the flux of substrate and product is controlled by the entire pathway and not just the single target enzyme. The target enzyme's product concentration is determined by kinetic parameters for the enzyme and available substrate concentration.
The conditions under which the enzymes are studied (in vitro) and operate (in vivo) are very different.
- In vitro (in the lab), the enzyme is held at a constant concentration while the substrate is varied (i.e., the substrate concentration is the independent variable). The velocity is determined by the substrate concentration. When inhibition is studied, the substrate is varied while the inhibitor is held constant at several different fixed concentrations.
- In vivo (in the cell), the velocity might be held at a relatively fixed level, with the substrate concentration determined by the velocity. The substrate can't build up at the enzyme to avoid a bottleneck in flux, so the enzyme processes it in a steady state fashion to produce the product as determined by the Michael-Menten equation.
What happens when an inhibitor is added in vivo? Let's assume that the enzyme is running at v = Vm/2. How might in vivo inhibition plots look at constant velocity (for example, v=Vm/2) when both I and S can vary, and in which S for an enzyme in the middle of a pathway is determined by v?
The equations and graph below show the ratio of S/Km vs I/Kix for inhibition at constant v, a condition encountered when an enzyme in a metabolic pathway is subject to flux controls imposed by the entire pathway. The x-axis reflects the ratio of inhibitor concentration to its inhibition constant. Likewise, the y-axis reflects the relative amount of substrate compared to its Km. The graph for in vivo competitive inhibition is linear, but it "blows up" for uncompetitive inhibition, as shown in Figure \(\PageIndex{15}\).
Here are the derivations used to produce the graphs in Figure \(\PageIndex{13}\).
A derivation of the competitive and uncompetitive inhibition in vivo when v = constant
Here it is!
- Derivation
-
Competitive Inhibition at constant velocity v:
Let's start with the equation of competitive inhibition.
\begin{equation}
\mathrm{v}=\frac{\mathrm{V}_{\mathrm{M}} \mathrm{S}}{\mathrm{K}_{\mathrm{M}}\left(1+\frac{\mathrm{I}}{\mathrm{K}_{\mathrm{is}}}\right)+\mathrm{S}}
\end{equation}Let
\begin{equation}
\left(1+\frac{\mathrm{I}}{\mathrm{K}_{\mathrm{is}}}\right)=\mathrm{y}
\end{equation}Then,
\begin{equation}
\begin{gathered}
v=\frac{V_M S}{K_M y+S} \\
v\left(K_M y+S\right)=V_M S \\
v K_M y+v S=V_M S \\
v K_M y=V_M S-v S=S\left(V_M-v\right) \\
K_M=\frac{S\left(V_M-v\right)}{v y}
\end{gathered}
\end{equation}which gives:
\begin{equation}
\begin{gathered}
\frac{S}{K_M}=\frac{v y}{\left(V_M-v\right)}=\frac{y}{\frac{V_M}{v}-1}=\frac{1+\frac{I}{K_{i s}}}{\frac{V_M}{v}-1}=\frac{1}{\frac{V_M}{v}-1}+\frac{\frac{I}{K_{i s}}}{\frac{V_M}{v}-1} \\
\frac{S}{K_M}=\frac{1}{\frac{V_M}{v}-1}+\left(\frac{1}{\frac{V_M}{v}-1}\right) \frac{I}{K_{i s}}
\end{gathered}
\end{equation}Note from the last equation that the graph of S/KM vs I/Kis is linear (at a fixed v), as shown in the above figure.
Uncompetitive Inhibition at constant velocity v:
Let's start with the equation of uncompetitive inhibition.
\begin{equation}
\mathrm{v}=\frac{\mathrm{V}_{\mathrm{M}} \mathrm{S}}{\mathrm{K}_{\mathrm{M}}+\mathrm{S}\left(1+\frac{\mathrm{I}}{\mathrm{K}_{\mathrm{ii}}}\right)}
\end{equation}Let
\begin{equation}
\left(1+\frac{1}{\mathrm{K}_{\mathrm{ii}}}\right)=\mathrm{y}
\end{equation}then:
\begin{equation}
\begin{gathered}
v=\frac{V_M S}{K_M+S y} \\
v\left(K_M+S y\right)=V_M S \\
v K_M=V_M S-v S y=S\left(V_M-v y\right)
\end{gathered}
\end{equation}which gives:
\begin{equation}
\frac{S}{K_M}=\frac{v}{V_M-v y}\left(\frac{\frac{1}{v}}{\frac{1}{v}}\right)=\frac{1}{\frac{V_M}{v}-y}=\frac{1}{v-1-\frac{I}{K_{i i}}}
\end{equation}This graph is not a linear function of I/Kii as it was for in vivo competitive inhibition.
- for competitive inhibition, the graph of S/KM is a linear function of I/Kis
- for uncompetitive inhibition, the graph of S/KM is NOT a linear function of I/Kii but rather "blows up" to infinity.
These graphs and associated equations are dramatically different from the very similar forms of inhibition equations and curves for in vitro inhibition at varying S and different fixed values of inhibitor. Consider the uncompetitive graph and equation. In the absence of an inhibitor, if S=Km, then Vm/v = 2, so the calculated value from the equation above of S/Km = 1. The y-intercept of the graph above is 1 for uncompetitive (and competitive) inhibition. If I is allowed to increase to a value of Kii (so I/Kii = 1), again at constant v=Vm/2, then the right-hand side goes to infinity.
In a linked series of reactions, if the middle reaction is inhibited, the substrate for that enzyme builds, whether the inhibition is competitive or uncompetitive. With competitive inhibition, the substrate concentration can be raised to meet the requirements of the enzyme. However, as the above figure shows, this can't happen for uncompetitive inhibition since, as more substrate accumulates, the reaction reaches a point where the steady state is lost.
Obviously, this limiting case can't be realistically reached, but it does suggest that uncompetitive inhibitors would be more effective in vivo in controlling a metabolic pathway than competitive inhibitors. Cornish-Bowden argues that purely uncompetitive inhibitors are rare because of the degree of inhibition they can hypothetically produce (1986). Likewise, he suggests that medicinal chemists should synthesize uncompetitive inhibitors if their goal is to maximally inhibit a metabolic pathway under the kind of flux control described above. Although it is more difficult to synthesize a purely uncompetitive inhibitor (as it can't be easily modeled after the structure of a natural ligand that binds to the active site and are competitive inhibitors), he notes that synthesizing mixed (and noncompetitive) inhibitors whose Kii values are of reasonable size compared to their Kis values, would be one approach.
Move the sliders on the interactive graph below to show how the graphs change. Disregard the graph in the right lower quadrant, as that location would require negative values of either S or K.
Inhibition by Temperature and pH Changes
From 0 to about 40-50 oC, enzyme activity usually increases, as do the rates of most reactions in the absence of catalysts. (Remember the general rule of thumb that reaction velocities double for each increase of 10 oC). At higher temperatures, the activity decreases dramatically as the enzyme denatures. These features are illustrated in Figure \(\PageIndex{16}\).
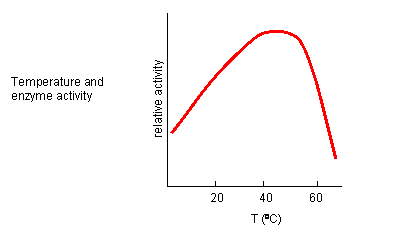
Likewise, pH has a marked effect on the velocity of enzyme-catalyzed reactions as illustrated in Figure \(\PageIndex{17}\).
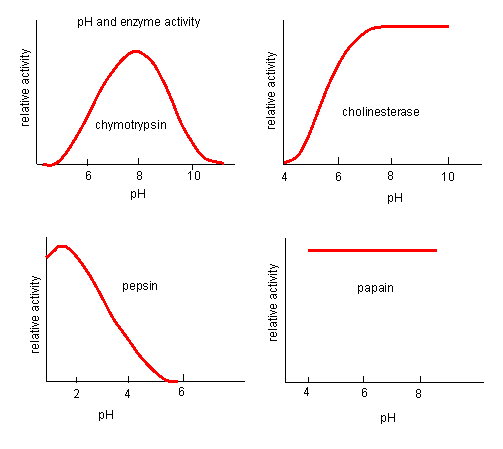
Think of all the things that pH changes might affect. It might
- affect E in ways to alter the binding of S to E, which would affect Km
- affect E in ways to alter the actual catalysis of bound S, which would affect kcat
- affect E by globally changing the conformation of the protein
- affect S by altering the protonation state of the substrate
The easiest assumption is that key side chains necessary for catalysis must be in the correct protonation state. Thus, a sidechain, with an apparent pKa of around 6, must be deprotonated for optimal activity of trypsin, which shows increases in activity with pH centered at pH 6. Which amino acid side chain would be a likely candidate?
Table \(\PageIndex{1}\) below shows how pH effects on enzyme kinetics can be modeled at the chemical and mathematical level.
|
\begin{equation} |
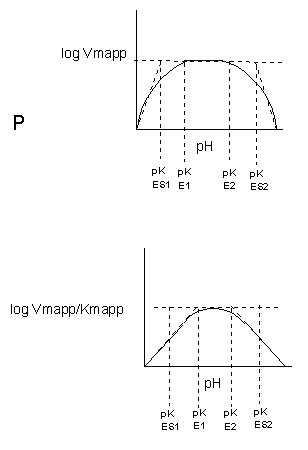 |
Summary
Chapter Summary: Enzyme Inhibition – From Irreversible Covalent Modifications to Reversible Inhibition Mechanisms
This chapter explores the diverse strategies by which enzymes can be inhibited, emphasizing the molecular basis of both irreversible and reversible inhibition. Understanding these mechanisms is crucial for appreciating how enzyme activity is regulated in biological systems and for the design of therapeutic inhibitors.
-
Irreversible Covalent Inhibition
The chapter begins by discussing how enzyme activity can be permanently abolished through irreversible modifications. These modifications, whether induced by extremes of pH, high temperature, or covalent binding of small molecules (e.g., iodoacetamide modifying critical cysteine residues), result in structural changes that render the enzyme inactive. Techniques to identify essential catalytic side chains using protecting groups are also introduced. -
Reversible Inhibition Mechanisms
The discussion then shifts to reversible inhibition, which is subdivided into several mechanistic types:- Competitive Inhibition:
Substrate and inhibitor compete for the same active site on the enzyme. Kinetic analysis shows that while VMV_M remains unchanged, the apparent KM increases (i.e., KM app=KM(1+I/Kis). Graphical representations (e.g., Lineweaver-Burk plots) illustrate that the lines intersect on the y-axis. - Uncompetitive Inhibition:
The inhibitor binds exclusively to the enzyme–substrate complex, reducing both VM and KM proportionally, which is reflected as parallel lines in Lineweaver-Burk plots. - Noncompetitive and Mixed Inhibition:
When an inhibitor binds both to free enzyme and the ES complex (with equal or different affinities), the kinetic effects differ. In pure noncompetitive inhibition, VM decreases without changing KM, while mixed inhibition shows changes in both parameters. These distinctions are crucial for understanding enzyme regulation and designing inhibitors.
- Competitive Inhibition:
-
Graphical and Mathematical Analysis of Inhibition
The chapter emphasizes the use of various kinetic plots (e.g., Michaelis-Menten, Lineweaver-Burk, and progress curves) to analyze how inhibitors affect enzyme kinetics. Interactive graphs and simulations illustrate how changing inhibitor concentrations and their dissociation constants (e.g., Kis and Kii) influence parameters like KM and VM, as well as the overall reaction velocity. -
In Vivo versus In Vitro Considerations
The text contrasts inhibition studies conducted in vitro—where enzyme and substrate concentrations are controlled—with the complex, flux-driven environment in vivo. It discusses how metabolic pathways influence enzyme kinetics and how different inhibition mechanisms may have distinct effects under cellular conditions. For example, while competitive inhibitors can be overcome by increasing substrate concentration in vitro, uncompetitive inhibitors may exert more pronounced effects in vivo by preventing the accumulation of substrate. -
Environmental Effects on Enzyme Activity
Finally, the chapter reviews how external factors such as temperature and pH influence enzyme activity. Moderate increases in temperature typically enhance reaction rates, whereas excessive heat leads to irreversible denaturation. Similarly, pH changes can alter the protonation state of key catalytic residues, thereby affecting both substrate binding and catalytic turnover.
In summary, this chapter provides a comprehensive understanding of enzyme inhibition by detailing both irreversible covalent modifications and reversible inhibition mechanisms. It integrates mathematical derivations with graphical analyses and computational models to illustrate how inhibitors modulate enzyme kinetics. These insights are essential for the rational design of inhibitors in drug discovery and for a deeper understanding of metabolic regulation in living systems.




.png?revision=1&size=bestfit&width=390&height=330)
.png?revision=1&size=bestfit&width=353&height=323)
.png?revision=1&size=bestfit&width=372&height=299)