Module 2.2: Water and Hydrogen Bonds
- Page ID
- 24649
learning objective
-
Explain the molecular structure of Water.
-
Identify hydrogen bond donors and acceptors.
-
Predict the strength of hydrogen bonds based on geometry.
-
Predict the solubility of compounds in water.
Polar Bonds and Molecules
A bond is considered to be polar if there is a significant difference in the electronegativities of the participating atoms. Electronegativity is a measure of an atom's attraction for electrons; a more electronegative atom will pull some electron density from other bonded atoms that are more electropositive. The following table gives the electronegativities of atoms that are common in biochemistry. These values can be used to estimate the partial charge on atoms in molecules. For example, since the electronegativity of hydrogen is smaller than C, S, N, and O, any bonds between hydrogen and these atoms will result in a partial positive charge on the hydrogen and a negative charge on the other atom. The larger the difference in electronegativity, the larger the difference in partial charges.
| Atom | Electronegativity |
|---|---|
| H | 2.20 |
| C | 2.55 |
| S | 2.58 |
| N | 3.04 |
| O | 3.44 |
The dipole moment, \(\mu\), is defined by the following equation:
where \(q\) is the charge on the atom and r is the distance to the center of mass.
\[\mu=\sum \space_{All\space atoms} \space qr\nonumber\]
A polar molecule will have an overall net dipole moment. It is possible for a non-polar molecule to have polar bonds. For example, carbon dioxide (O=C=O) contains two polar bonds, but the dipole moment of one bond cancels the other, leading to no net dipole and therefore a non-polar molecule.
The ammonia molecule (NH3) has three identical polar N-H bonds that are equally spaced around the nitrogen atom. Ammonia has a net dipole moment of 1.4 D, similar to that of water (1.85 D).
Based on this information, do you think ammonia is a planer molecule or not? Briefly justify your response.
- hint
-
What orientations of the individual dipole would give rise to a net dipole of zero?
- Answer
- Ammonia is not planer. If it were planer than the dipole moments associated with each N-H bond would cancel, giving a net dipole of zero.
Structure of Water
- Oxygen has the following electronic configuration: \(1s^22s^22p^4\).
- In water, the 2s and the three 2p orbitals form four \(sp^3\) hybrid orbitals.
- These orbitals are tetrahedral in their orientation, however, the ideal bond angle of \(109^{\circ}\) is distorted to \(104.5^{\circ}\).
- The orbitals are populated such that two orbitals are filled and two contain one electron each.
- The filled orbitals cannot form bonds and are called lone pairs of electrons.
- The half-filled orbitals participate in the formation of a sigma bond between oxygen and hydrogen.
- "Bent" water molecule generates a permanent dipole moment, making water a polar solvent.
learn by doing
Water and Ice
Drag the model with your mouse to rotate it in 3D space. Use your right mouse button or "control" click on the Jmol to bring up a menu of options for manipulating the model.
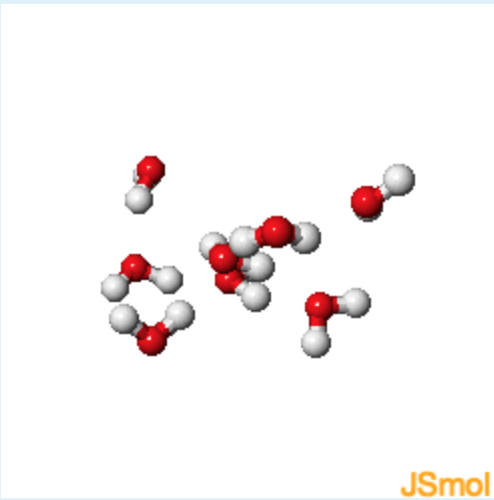
Water-Liquid
Ice
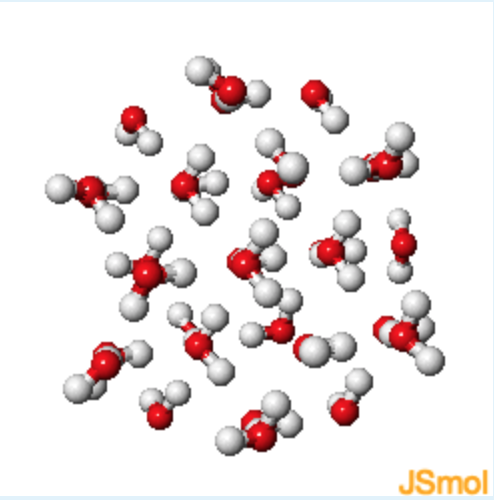
1. Identify the following atoms based on the colors from the figures above
- hint
-
focus on a single water molecule.
- Answer
-
Red: Oxygen; White: Hydrogen
2. Which set of atoms on different molecules will have a greater distance between them? Hydrogen to Hydrogen or Hydrogen to Oxygen?
- hint
-
check the actual distance
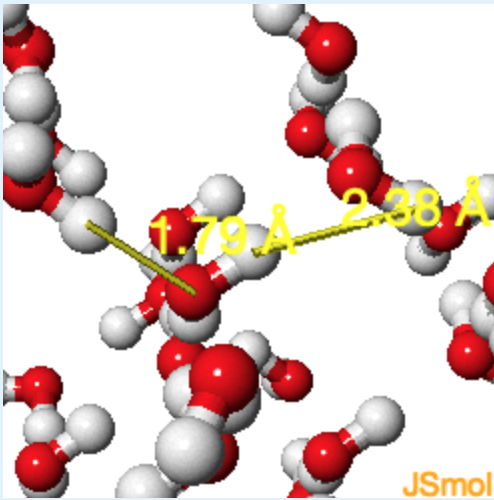
- Answer
-
Hydrogen to Hydrogen (The same positive partial charges of the hydrogen atoms causes them to slightly repel each other. The opposite partial charges of the oxygen and hydrogen atoms cause them to attract each other.)
3. Describe the relative orientation of the hydrogen atoms (white) with respect to the oxygen atoms (red). How does this orientation differ between water and ice?
What is the physical basis for this orientation?
- hint
-
It may be helpful to rotate the molecules to see the relative positions of the atoms.
- Answer
-
The hydrogen atoms are generally found to be close in space to the oxygens. The distances between the hydrogen and the oxygen are more uniform in ice than in water. The hydrogens preferentially orient in this manner because they have a partial positive change and the oxygen has a partial negative charge.
4. Compare liquid water and ice, which seems to have the lower density, and why?
- hint
-
view the physical property of ice that causes this phenomena.
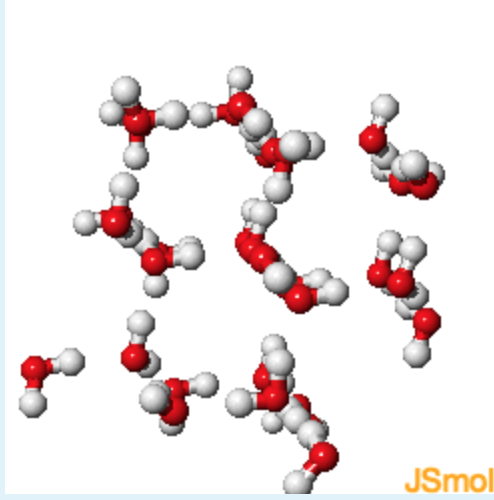
- Answer
-
Ice has the lower density (that is why it floats) due to hexagonal channels in the ice. The hexagonal channels form to optimize hydrogen bonding in solid water.
a. H-bonds are stable because of electron sharing across the bond (i.e. a weak covalent bond) and an electrostatic attraction between:
- Electropositive hydrogen, attached to an electronegative atom is the hydrogen bond donor (i.e. NH)
- Electronegative hydrogen bond acceptor (e.g. the lone pairs of oxygen in the case of water, or C=O group of an amide)
b. Typical length: 1.8 Å (from hydrogen to oxygen, 2.7 Å from hydrogen to nitrogen)
c. Typical angle: \(180^{\circ}\) \(\pm\) \(20^{\circ}\)
d. Typical energy: 20 kJ/mole.
e. Number of hydrogen bonds depend on temperature, 4/molecule at 0\(^{\circ}C\).
Biochemical Significance of Hydrogen Bonds:
- In ice, the hydrogen bonds cause the formation of cavities in the ice, lowering the density of the solid.
- In liquid water, the hydrogen bonds persist, and are transiently formed on a time scale of ~nano seconds, generating small short-lived clusters of "ice" in liquid water.
- Hydrogen bonds are present over a wide temperature range.
- The hydrogen bonds in water allow water to absorb heat by breaking the hydrogen bonds without a large increase in temperature, giving water a high heat capacity.
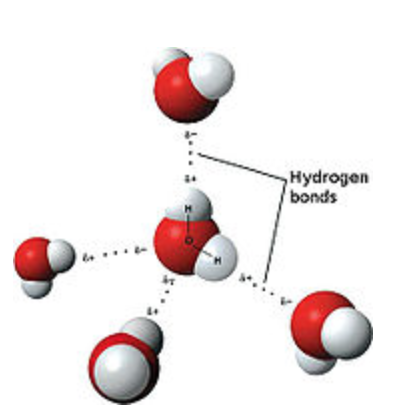
The 4 possible hydrogen bonds formed with a water molecule in ice. The number of hydrogen bonds formed/molecule in liquid water is less than four, and decreases as the temperature increases. At room temperature each water molecule forms on average approximately 3 hydrogen bonds.
learn by doing
1. Which of the following sets of atoms will not form a strong hydrogen bond?
a. C-H.....O-C
b. O-H....N
c. N-H....O
d. O-H....O
- hint
-
The donor hydrogen should be attached to a strongly electronegative atom.
- Answer
-
a. (the donor hydrogen is not attached to an electronegative atom.)
2. Which of the following hydrogen bonds would be the strongest (lowest in energy)?
a. An H to O distance of 1.0 A
b. An H to O distance of 2.0 A
c. An H to O distance of 2.5 A
- hint
-
The optimal distance between donor and acceptor depend on the balance between van der Waals forces and electrostatic forces.
- Answer
-
b. (this is very close to the optimal distance for the electrostatic interaction in hydrogen bonds.)
3. Which of the following hydrogen bonds would be the weakest?
a. An N-H...O angel of 150 degree
b. An N-H...O angel of 170 degree
c. An N-H...O angel of 180 degree
- hint
-
Hydrogen bonds are partially covalent.
Answer-
a. ( hydrogen bonds are also partially covalent and optimal overlap of the shared electrons occurs when they are linear.)
Solvation and Solubility
Hydrophobic compounds do not contain polar atoms and therefore cannot interact with water via hydrogen bonding. Consequently, solvated hydrophobic compounds cause the formation of an ordered shell of hydrogen bonded water molecules. Removal of the solvated hydrophobic compound will release these water molecules, increasing the entropy of the solvent. This favorable increase in the entropy of the solvent drives the hydrophobic molecules from the aqueous phase. The hydrophobic effect is responsible for the spontaneous formation of a number of important biological structures, such as:
- Proteins
- cell membranes
- Interaction of small molecules with larger proteins, such as substrates with enzymes.
Hydrophillic compounds contain polar atoms, such as nitrogen or oxygen. Consequently, they can form hydrogen bonds with water. The formation of hydrogen bonds is energetically favorable, thus hydrophillic compounds readily dissolve in water.
Ionic compounds are readily solvated by water. There are two factors that favor a dissolved solution of ions over the crystalline form.
- Increase in entropy of the ions. A crystal is highly ordered, with low entropy. Dissolved ions are dispersed throughout the solution, a high entropy state.
- Electrostatic shielding. The force between two charged particles is: The force depends on the distance between the two charges and the dielectric constant (D) of the media. A high dielectric constant, such as that found in water, is important because the forces between charges are attenuated or reduced. Making it less favorable to have an electrostatic interaction between the positive and negative ions.
\[F=\frac{1}{4 \pi \varepsilon_{o}} \frac{q_{1} q_{2}}{D r^{2}} \quad \varepsilon_{o}=8.854 \times 10^{-12} C^{2} / N m^{2}\nonumber\]
The dielectric constant is proportional to the dipole moment of the solvent, as the dipole moment increases, D, increases, as shown in the following table. A large dipole moment means that the solvent molecules can interact favorably with charged solute molecules.
| Compound | Dielectric Constant | Dipole Moment |
|---|---|---|
| Water | 79 | 1.85 |
| Methanol | 32 | 1.66 |
| Benzene | 2 | 0.00 |
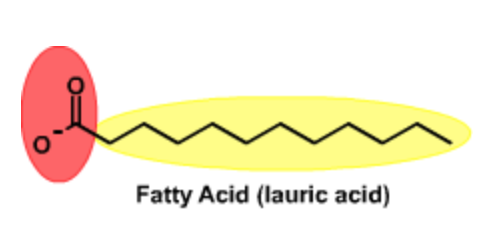
Amphipathic (or amphiphilic) compounds are both polar (or charged) and nonpolar. An example is a fatty acid, which has a charged carboxylate (red) and a non-polar hydrocarbon chain (yellow). These can form micelles if the nonpolar part is sufficiently large. Micelles are aggregates of amphipathic molecules that sequester the nonpolar part on the inside, much like the inside of an orange. Micelles will form spontaneous driven by the hydrophobic effect.
Review Quiz
DID I GET THIS
1. The partial negative charge at one end of a water molecule is attracted to the partial positive charge of another water molecule. This attraction is called:
a. a covalent bond.
b. a hydration shell.
c. a hydrogen bond.
d. a hydrophobic bond.
e. an ionic bond.
- Answer
-
c. (The statement is a fairly complete definition of the hydrogen bond; Hydration shell refers to organization of water molecules around a non-polar group; "Hydrophobic Bond" is not a real term, don't confuse it with a "hydrogen bond"; Partial charges cannot be involved in ionic bonds, only full charges can form ionic bonds.)
2. A hydrogen bond that is linear is more stable than one that is bent.
True
False
- Answer
-
True (A linear geometry is more stable due to the partial covalent nature of the hydrogen bond.)
3. On average, there are ____ hydrogen bonds per molecule in water at room temperature and _____ hydrogen bonds per molecule in ice.
- hint
-
Look carefully at the Jmol images and static picture of hydrogen bonds in ice and water.
- Answer
-
3; 4
4. Which of the following alcohols would be most soluble in water?
a. methanol (\(CH3OH\))
b. ethanol (\(CH_3CH_2CH_2OH\))
c. butanol (\(CH_3CH_2CH_2CH_2OH\))
d. octanol (\(CH_3(CH_2)_6CH_2OH\))
e. phenol (benzyl-OH)
- hint
-
All of these compound have the same polar group (-OH). In what way do they differ?
- Answer
-
a. (The alcohol with the least amount of non-polar character would be the most soluble.)
5. Hydrophobic interactions
a. are responsible for the surface tension of water
b. are stronger than covalent bonds
c. can hold two ions together
d. are the major force forming lipid (hydrocarbon) bilayers
- hint
-
Hydrophobic compounds do not contain polar atoms and therefore cannot interact with water via hydrogen bonding.
- Answer
-
d.

