Module 2.1: Introduction and Water Structure
- Page ID
- 24557
learning objective
- Provide a general overview of biochemistry based on component parts.
- Describe molecular orbitals.
- Characterize functional groups.
- Recognize chiral centers
Cell Theory
Every cell on Earth belongs to one of two categories:
- Prokaryotic cells
- Eukaryotic cells
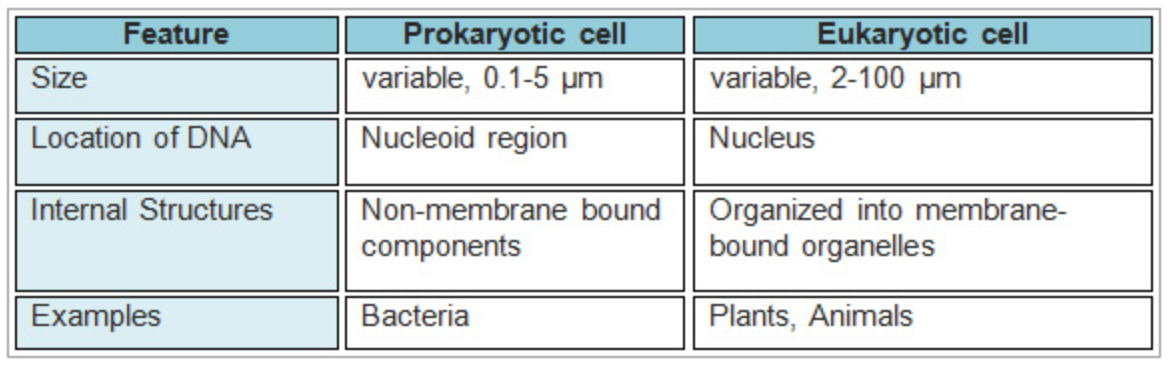
Prokaryotic cells were the first cells to appear on our planet. All prokaryotes alive today are unicellular (one-celled), and include bacteria (singular form is "bacterium") and archaea (singular form is "archaean"). Prokaryotes are small cells that don't have a nucleus or membrane-bound organelles.
Eukaryotic cells appeared after prokaryotes. The main difference between the two is that eukaryotes have a central control structure, called the nucleus (plural form is "nuclei"), where DNA is housed. In prokaryotes, the main DNA molecule (bacterial chromosome) is present in a region called the nucleoid, but the nucleoid lacks a surrounding membrane. Smaller DNA molecules called plasmids can be also found in prokaryotes. Prokaryotic DNA is circular, in contrast to the linear structure of eukaryotic DNA.
Both eukaryotic and prokaryotic cells have a cell or plasma membrane, which surrounds and defines the inner environment of the cell. The cell membrane is made of a phospholipid bilayer containing a variety of proteins and additional components. The cell membrane is responsible for mediating interactions between the cell and its environment and permits certain molecules to enter the cell.
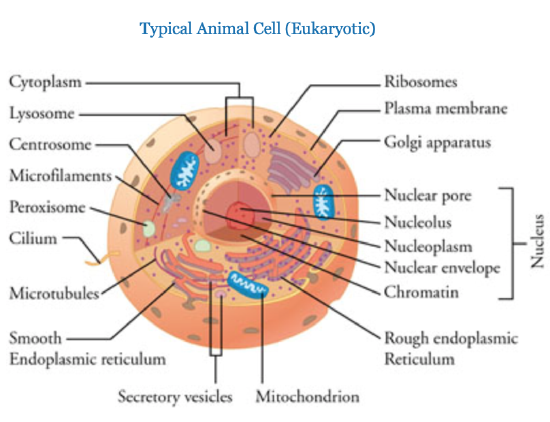
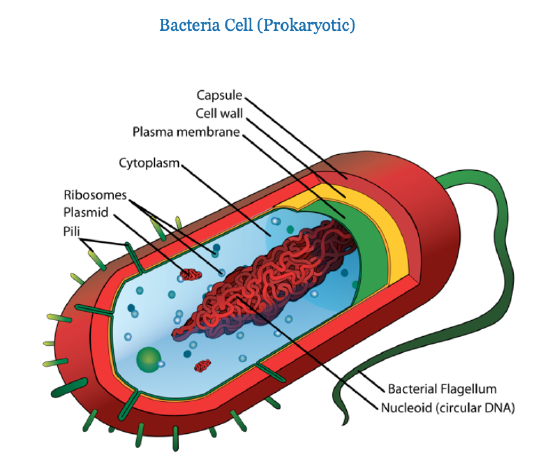
Note: these images are not drawn to scale. A typical eukaryotic cell is 10 times bigger than the typical prokaryotic cell.
Prokaryotic cells have a simpler structure than eukaryotic cells, and they range in diameter from 0.1 to 5.0 µm (micrometers). Most prokaryotes have a protective layer called the cell wall that is made of peptidoglycan, which is a combination of polysaccharides and amino acids. Although eukaryotic plant cells also have a cell wall, it has a different molecular structure. Prokaryotes, like prokaryotic cells, also have a cell membrane and cytoplasm. Many prokaryotes also have external appendages such as a flagellum. The cytoplasm contains the DNA and the ribosomes, where protein synthesis takes place. Several types of RNA are involved in the process of protein synthesis, and ribosomal RNA (rRNA) is the main component of ribosomes. Ribosomes can be found free from membranes, but they bind to membranes when synthesizing exported proteins. Both prokaryotes and eukaryotes have ribosomes, but they are different.
Eukaryotic cells have internal membrane bound compartments, called organelles, that perform specialized functions for the cell. These compartments and their functions are as follows:
| Organelle | Function |
|---|---|
| nucleus | Contains DNA in the form of chromosomes. |
| smooth endoplasmic reticulium | Site of lipid synthesis |
| rough endoplasmic reticulium | First part of the protein secretory pathway |
| golgi | Part of the protein secretory pathway. |
| Mitochondria | Site of energy production |
| Lysosome | Destruction of unwanted material. |
Comparison of cell sizes
Relative sizes of cells and their contents. Notice that the scale shown is logarithmic.
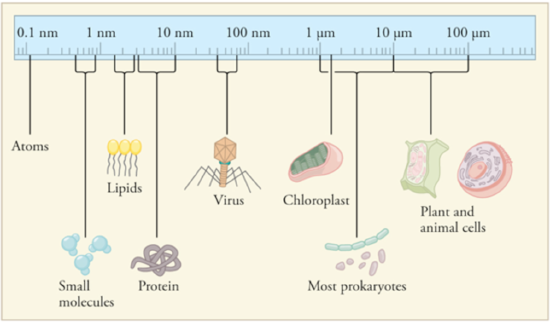
Relative sizes of cells and their contents. Notice that the scale shown is logarithmic.
Information flow in Living Organisms
The repository of genetic information in all organisms in a double stranded polymer called DNA. The DNA contains all of the information required for the cell to function, encoded in the order of four nucleotide bases, A, G, C, or T. In order for this information to be utilized it is necessary to copy the sequence of bases from DNA to RNA in a process called transcription. RNA differs from DNA in several key points: i) it is usually a single stranded molecule, ii) the T base in DNA is replaced by U. There are many functinally different RNA molecules. A class of RNA molecules, called mRNA (messenger RNA) are utilized by ribosomes to generate proteins, in this case the sequence of the nucleobases in the mRNA are translated to a sequence of amino acids in the protein.
Most viruses, which are technically not living because they cannot reproduce on their own, also utilize DNA as their genetic material. Some viruses use RNA. Some viruses copy the RNA directly to new RNA molecules when new viruses are made. Other viruses, called retroviruses, copy the RNA first to DNA (retro, or backwards) and then back to RNA. One of the more notable retroviruses is the HIV (human immunodeficiency virus) that causes acquired immunodeficiency syndrom (AIDS).
Understanding biochemistry requires that you understand the basic functions of the cell, the differences between eukaryotic and prokaryotic cells, and fundamental features of genetics. Take the following ungraded quiz to determine whether you need to review this material.
checkpoint
1. Ribosomes
a. are the site of photosynthesis
b. are the site of protein synthesis
c. are never bound to membranes
d. replicate DNA
- hint
-
The purpose of this ungraded quiz is for you to determine if you need to review before beginning this Biochemistry material. Answering these four questions will help you decide.
- Answer
-
a
2. Mitochondria
a. play no role in aerobic metabolism
b. are the site of photosynthesis in green plants
c. oxidize fuel to produce energy (ATP).
d. do not exist in animals
- Answer
-
c
3. Which of the following are found in both prokaryotic and all eukaryotic cells.
a. cell wall, cell membrane
b. endoplasmic reticulum, DNA replication.
c. golgi, periplasmic space
d. ribosomes, cell membrane
- Answer
-
d (All cells have ribosomes for protein synthesis and a cell membrane.) (a. Cell walls are found only in prokaryotes and plants. b. Prokaryotes lack internal membrane systems, such as the endoplasmic reticulum. c. Prokaryotes lack internal membrane systems, such as the golgi. Eukaryotes do not have a periplasmic space.)
4. DNA, the genetic coding material for most organisms, is usually first converted to this type of molecule during protein synthesis.
a. protein
b. DNA
c. polysaccharide
d. RNA
- Answer
-
d (The central "dogma" is DNA -> mRNA -> protein. In some RNA viruses the direction of information is RNA->DNA->RNA->protein.)
Introduction to Biochemistry
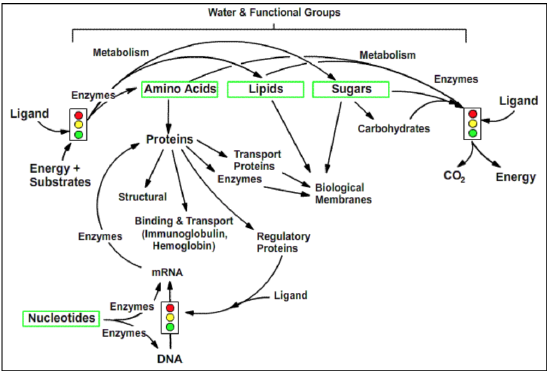
Overview of Central Themes in Biochemistry. Simple building blocks, such as amino acids, are used to generate complex biochemical structures, such as proteins. Proteins play diverse roles in the cell, including structural, transport, and catalysis. The information for the generation of such complex structures is encoded in nucleic acids. The flow of this information is regulated in a complex fashion. The entire process is fueled by metabolic processes that generate energy from organic energy sources and use this energy to synthesize complex molecules.
Chemical Bonding and Biochemical Structures
The elements that are commonly found in biochemical molecules are among the most abundant on earth, this includes carbon, oxygen, nitrogen, sulfur, and phosphorous.
Atoms are made up of subatomic particles: protons, neutrons, and electrons. Protons and neutrons are at the center of the atom and have a mass of 1 atomic mass unit (a.m.u) each. Each proton has a positive charge (+1), while neutrons are neutral (they carry no charge). Each electron has a negative charge (-1) and zero mass. Two atoms that differ by the number of neutrons are called isotopes of the same element.
One characteristic of the atoms of the major elements is that they are able to form molecules through formation of covalent bonds with other atoms.
- Covalent bonds
- (definition)Covalent bonds represent the sharing of the electrons (negatively charged subatomic particles between atoms.) The number of covalent bonds that can form is dictated by the number of unpaired electrons in the outer valence shell of the atom.
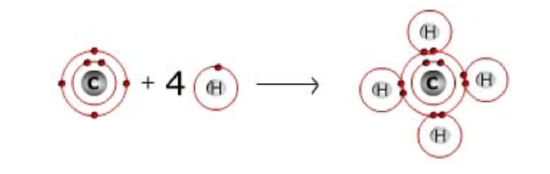
Each atom in a molecule will complete its outer shell of electrons, which is 2 for hydrogen, and 8 for second row elements (e.g. C, N and O). The valence shells for each of the biologically relevant elements are highlighted in the periodic table below.
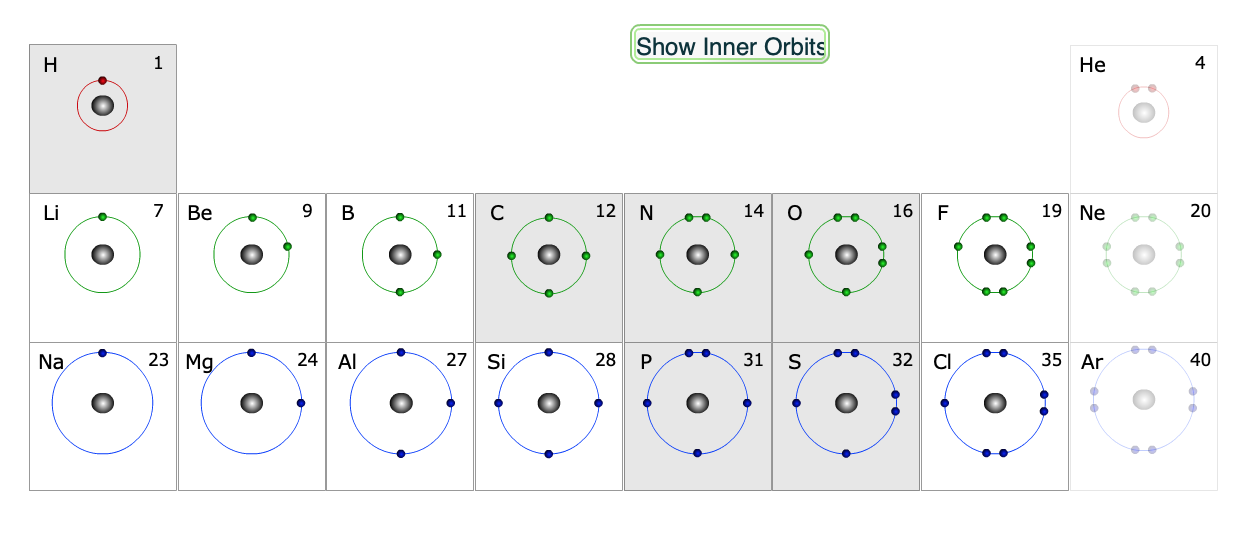
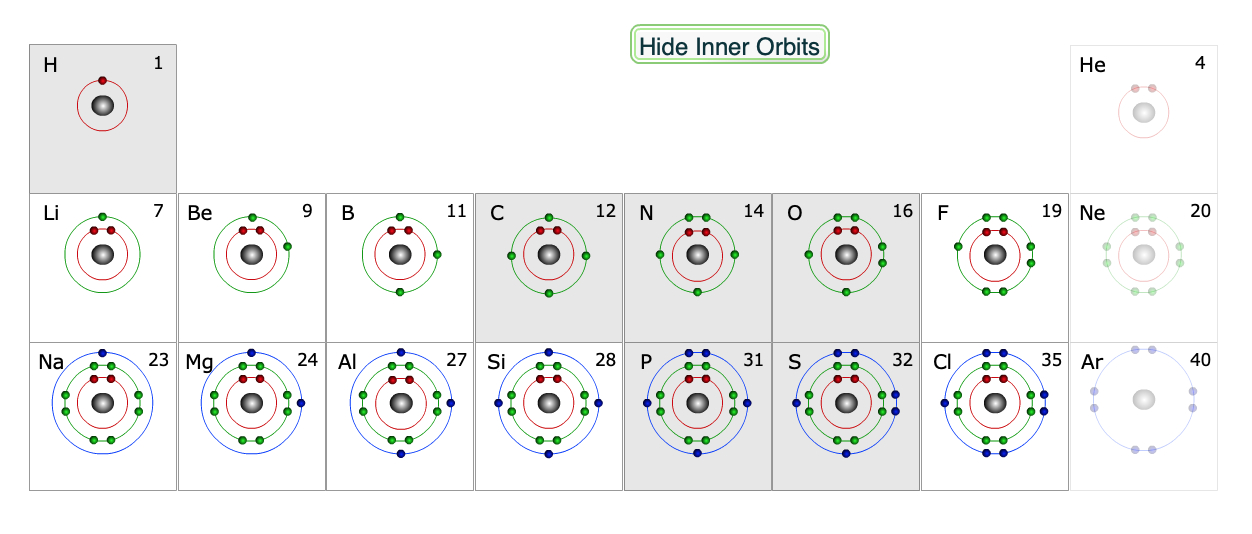
Only the valence shells are shown. The six shaded elements have unpaired electrons and readily form covalent bonds.
The electron shells are divided into atomic orbitals. Each atomic orbital holds at most 2 electrons. The s orbital is spherically symmetric. The three p orbitals (\(p_x, p_y, p_z\)) are bi-lobed, have a defined direction in space, and hold a total of 6 electrons. The \(1^{st}\) shell consists of the 1s orbital, and can hold 2 electrons. the \(2^{nd}\) shell contains the 2S and the \(2p_x, 2p_y\), and \(2p_z\) orbitals and can thus hold 8 electrons. The \(3^{rd}\) shell contains the 3S, \(3p_x, 3p_y, 3p_z\), and the five 3d orbitals. Since the 3d orbitals are very high in energy, it is reasonable to consider that the 3rd shell really contains 8 electrons as well. For most elements that are found in biological systems it is sufficient to consider the \(1^{st}\) shell, the \(2^{nd}\) shell, and the 3S and 3P orbitals as the third shell
| Atom | Electronic Configuration | Number of bonds | Hybrid Orbitals |
|---|---|---|---|
| H | \(1s^1\) | 1 | |
| C | \(1s^2 2s^2 2p^2\) | 4 | \(3\times sp^2 + pz\space or 4\times sp^3\) |
| N | \(1s^2 2s^2 2p^3\) | 3 (4 when protonated) |
\(3\times sp^2 + pz\space or 4\times sp^3\) |
| O | \(1s^2 2s^2 2p^4\) | 2 (3 when protonated) |
\(3\times sp^2 + pz\space or 4\times sp^3\) |
| S | \(3s^23p^4\) | 2 (usually) | |
| P | \(3s^23p^3\) | 5 |
Carbon, nitrogen, and oxygen, usually form hybrid orbitals, that show a mixture of s and p character, e.g. \(sp^3\)hybrid orbitals, as discussed below.
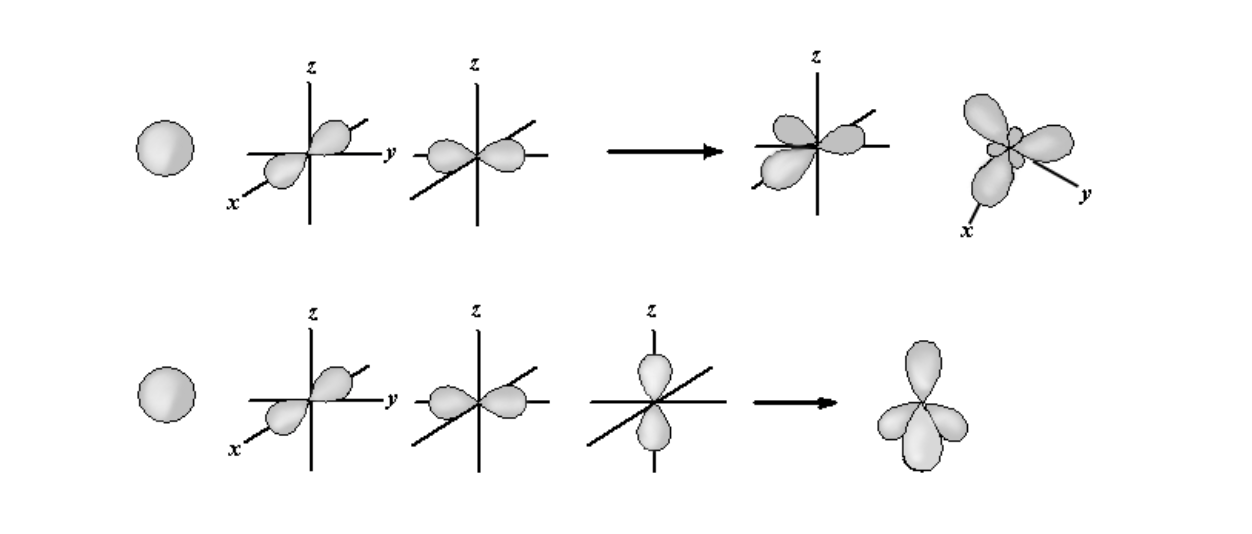
Generation of Hybrid Atomic Orbitals. The top section shows the generation of three \(sp^2\) orbitals from an s orbital and two p orbitals. The three \(sp^2\) orbitals all lie in the same plane and are 120\(^{\circ}\) from each other. The bottom section show the generation of four \(sp^3\) orbitals from an s orbital and three p orbitals. The resultant \(sp^3\) orbitals form a tetrahedron, with an angle of 109\(^{\circ}\) between each orbital.
Covalent bonds indicate the sharing of electrons between atoms. Usually two half-filled orbitals combine to form the bond. Hence hydrogen forms one bond while carbon forms four.
Chirality
When a molecule cannot be superimposed on its mirror image, it is said to be chiral. Biochemical processes are often able to distinguish between chiral molecules, thus it is important to be able to identify if a compound is chiral or not. Carbon, when sp3 hybridized, is tetrahedral. If the four groups attached to the carbon are different, then the molecule containing that carbon is chiral, and the carbon itself is refereed to as a chiral center. Planer groups, such as carbon hybridized as sp2, are not chiral, because the plane of the molecule forms a mirror plane within the molecule itself, thus the mirror images are identical.
learn by doing
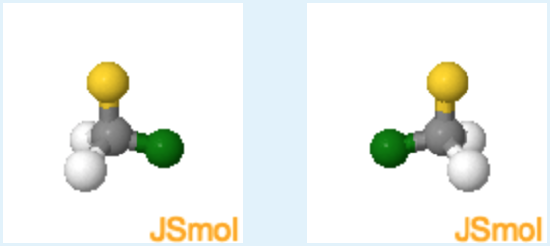
Chirality of Chloro-Fluoro Methane
Instructions: Chloro-fluoro methane and its mirror image are shown below. Please answer the question using these two structures.
1. How many different groups are attached to the central carbon atom?.
a. 1
b. 2
c. 3
d. 4
- hint
-
Different atoms are color coded, carbon grey, hydrogen white, chlorine green and fluorine yellow, how many different colors are present?
- Answer
-
c. 3
2. Based on your answer to the first question - should you be able to superimpose these two mirror images? Yes or No?
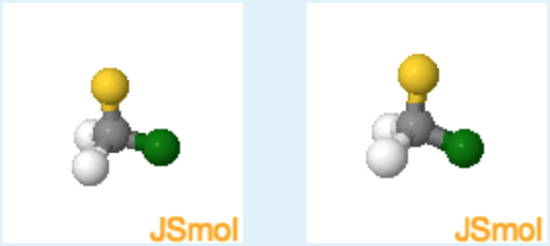 (orient)
(orient) 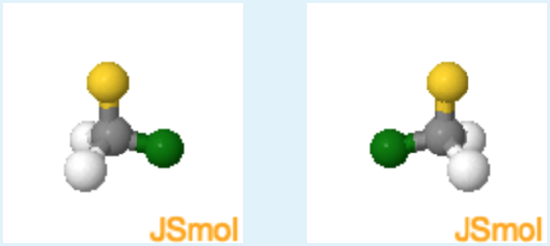
- hint
-
See if you can rotate one of the molecules to make it appear like the other.
- Answer
-
Yes. (since there are only three different atoms, these two molecules are not chiral and it is possible to superimpose their mirror images.)
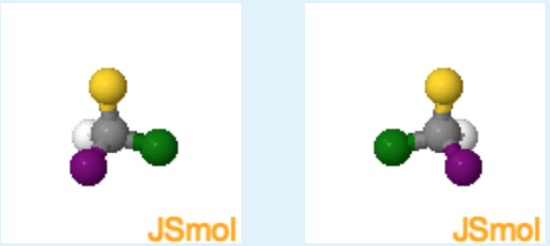
Chirality of Chloro-Fluoro-Bromo Methane
Instructions: One hydrogen has been replaced by bromine (colored purple). One form of the molecule is shown on the left and it's mirror image is on the right. Use these two structures to answer the question on the right.
1. Is it possible to superimpose one molecule on to its mirror image?
- hint
-
Try rotating one of the molecules to see if you can make it look like its mirror image.
- Answer
-
No. Because the carbon is bound to four different atoms it is now a chiral center. The mirror images cannot be superimposed.
Functional Groups
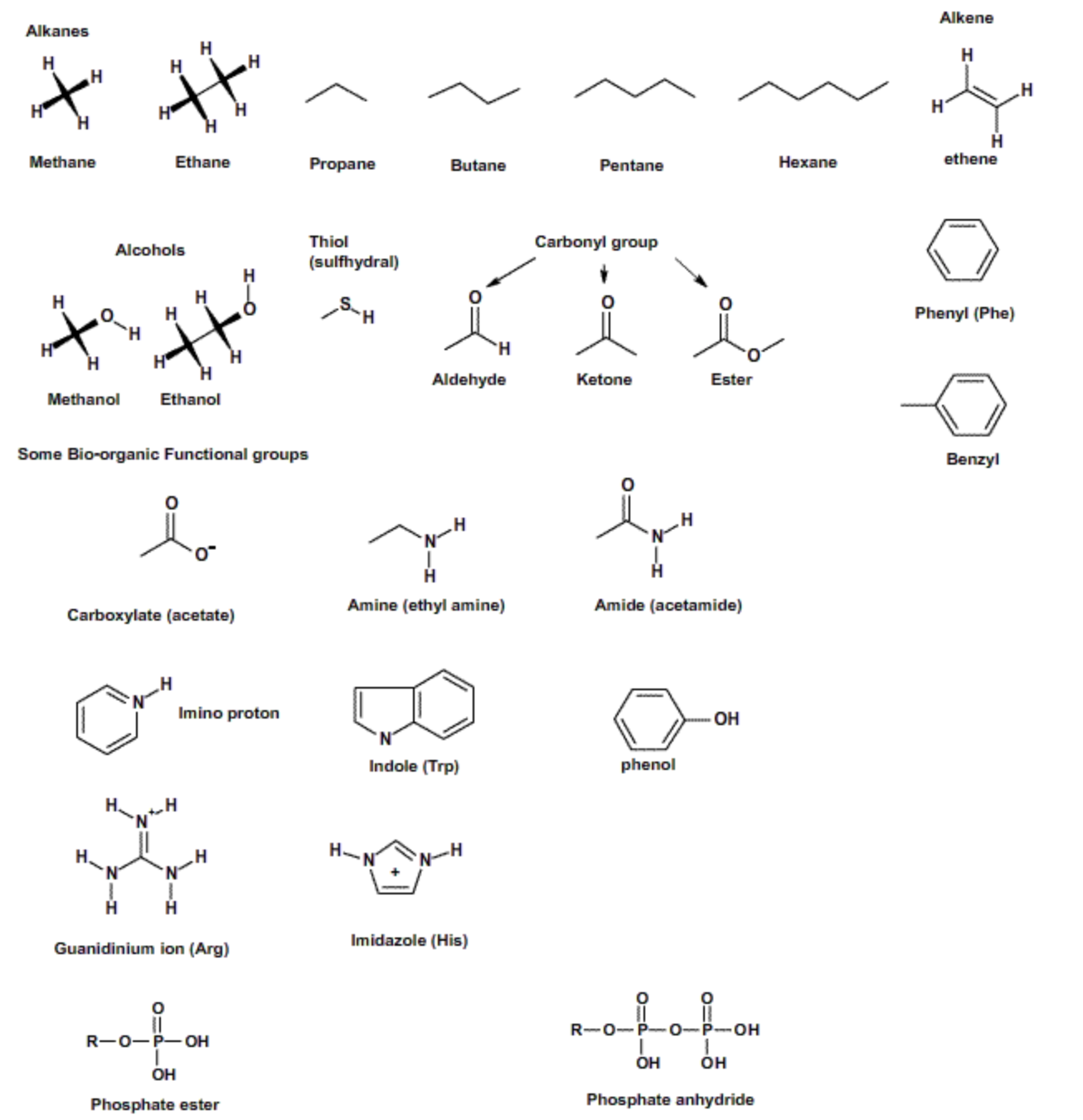
Important Functional Groups in Biochemistry. Alkanes and benzene aromatic rings do not contain polar atoms (e.g. N and O), and therefore do not interact with water, a polar solvent. Consequently they usually leave the aqueous phase. This hydrophobic behavior is very important in the self-assembly of many biochemical structures, such as proteins and membranes. The remaining compounds have some polar atoms and thus interact with water to a greater extent. Imidazole is found in the amino acid Histidine and plays an important role in the catalytic activity of many enzymes. The guanidinium group is found on the amino acid arginine and the indole group is found in the amino acid tryptophan.
Review Quiz
DID I GET THIS
Bonding and Functional Groups
1. Most (but not all) of the important functional groups on biological molecules
a. contain oxygen and(or) nitrogen and are acidic.
b. contain oxygen and a phosphate.
c. contain nitrogen and a phosphate.
d. contain oxygen and(or) nitrogen and are polar.
e. contain oxygen and(or) nitrogen and are nonpolar.
- hint
-
The purpose of this ungraded quiz is for you to determine if you need to review before beginning this Biochemistry material. Answering these four questions will help you decide.
- Answer
-
d (a. Partially correct, except they need not be acidic; b. Incorrect. Nitrogen is more common than phosphate. c. Incorrect. Oxygen is more common than phosphate. e. Incorrect. Oxygen and nitrogen, because of their high electronegativity are involved in polar bonds.)
2. Functional groups that are responsible for protein folding and membrane structure are:
a. ketone groups
b. alcohols
c. hydrocarbon
d. aldehydes
e. thiols
- hint
-
The polar oxygen atoms on water don't like to interact with these non-polar atoms, forcing the formation of structures which bury these atoms.
- Answer
-
c (Protein folding is driven by the hydrophobic effect which tends to bury non-polar regions. Hydrocarbon groups are non-polar and will drive folding due to Hydrophobic Effect.)
3. An s orbital is _________ while a p orbital is ____________
a. complex, spherical.
b. spherical, bi-lobed.
c. bi-lobed, spherical.
d. spherical, imaginary.
e. spherical, donut shaped.
- hint
-
The electron density for these orbitals are shown on this course page.
- Answer
-
b (The shape of the orbital is important for understanding bonding - the p orbitals provide directionality.)
4. Which of the following atoms often forms hybrid orbitals in covalent compounds?
a. Carbon
b. Nitrogen
c. Oxygen
d. All of these atoms.
e. None of these atoms.
- Answer
-
d (Hybrid orbitals are more stable for all three of these atoms.)
5. The \(sp^2\) orbitals are separated by ______ while the \(sp^3\) orbitals are separated by _______ and are ________.
a. 109 degrees, 120 degrees, planer.
b. 120 degrees, 109 degrees, planer.
c. 180 degrees, 109 degrees, tetrahedral.
d. 120 degrees, 109 degrees, tetrahedral.
e. 180 degrees, 90 degrees, octahedral.
- Answer
-
d (The geometry of the hybrid orbitals is important in the structure of biological molecules.)
6. Which of the following compounds has a chiral center?
a. \(CH_4\)
b. \(CH_3Cl\)
c. \(CH_2Cl_2\)
d. \(CHClFI\)
- Answer
-
d (Carbon is forming bonds to four different groups.)