11.3: Defense Cells in the Tissue - Dendritic Cells, Macrophages, and Mast Cells
- Page ID
- 3268
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- State 3 different functions of macrophages in body defense.
- State the primary function of dendritic cells in body defense.
- Name the cells in the tissue whose primary function is to present antigen to naive T-lymphocytes.
- Name the cells in the tissue whose primary function is to present antigen to effector T-lymphocytes.
- State the primary function of mast cells in body defense.
Dendritic Cells
Most dendritic cells are derived from monocytes and are referred to as myeloid dendritic cells. They are located throughout the epithelium of the skin, the respiratory tract, and the gastrointestinal tract, as well as lymphoid tissues and organ parenchyma. In these locations, in their immature form, they are attached by long cytoplasmic processes. Upon capturing antigens through pinocytosis and phagocytosis and becoming activated by inflammatory cytokines, the dendritic cells detach from their initial site, enter lymph vessels, and are carried to regional lymph nodes. By the time they enter the lymph nodes, they have matured and are now able to present antigen to the ever changing populations of naive T-lymphocytes located in the cortex of the lymph nodes.
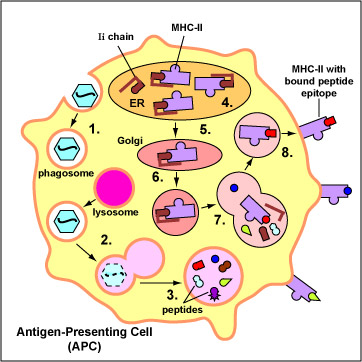
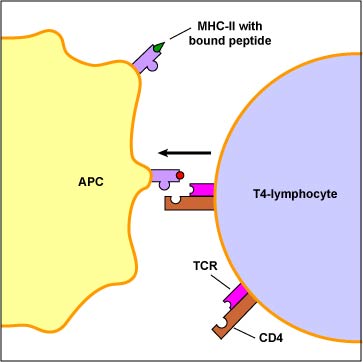
The primary function of dendritic cells is to capture and present protein antigens to naive T-lymphocytes. (Naive lymphocytes are those that have not yet encountered an antigen.) Dendritic cells engulf microorganisms and other materials and degrade them with their lysosomes. Peptides from microbial proteins are then bound to a groove of unique molecules called MHC-II molecules produced by macrophages, dendritic cells, and B-lymphocytes. The peptide epitopes bound to the MHC-II molecules are then put on the surface of the dendritic cell (Figure \(\PageIndex{1}\)) where they can be recognized by complementary shaped T-cell receptors (TCR) and CD4 molecules on naive T4-lymphocyte (see Figure \(\PageIndex{2}\)).
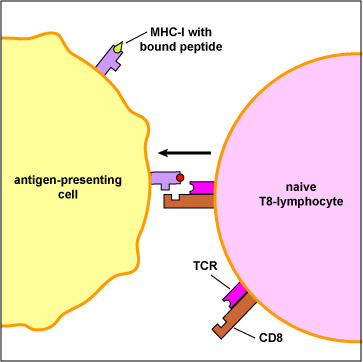
In addition, dendritic cells can bind peptide epitopes to MHC-I molecules and present them to naiveT8-lymphocytes. The MHC-I molecules with bound peptide on the dendritic cell are recognized by complementary shaped T-cell receptors (TCR) and CD8 molecules on naive T8-lymphocyte (Figure \(\PageIndex{3}\)).
A dendritic cell. (CC BY-SA 2.5; Judith Behnsen, Priyanka Narang, Mike Hasenberg, Frank Gunzer, Ursula Bilitewski, Nina Klippel, Manfred Rohde, Matthias Brock, Axel A. Brakhage, Matthias Gunzer - Source: PLoS Pathogens ).
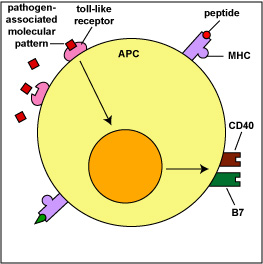
These interactions enable the T4-lymphocytes or T8-lymphocytes to become activated, proliferate, and differentiate into effector cells. This will be discussed in detail in Unit 6. Myeloid dendritic cells also use pattern-recognition receptors called toll-like receptors (TLRs) to recognize pathogen-associated molecular patterns or PAMPs (Figure \(\PageIndex{4}\)). The interaction of the PAMP with its TLR stimulates the production of co-stimulatory molecules that are also required for T-lymphocyte activation. Dendritic cells produce many of the same inflammatory cytokines as macrophages, such as tumor necrosis factor-alpha (TNF-alpha), interleukin-1 (IL-1), interleukin-6 (IL-6), and interleukin-8 (IL-8). They also can produce interleukin-12 (IL-12), a cytokine that can activate natural killer T-lymphocytes (NKT cells).
Another type of dendritic cell, the plasmacytoid dendritic cell, uses its TLRs to recognize viral PAMPs. This interaction results in the production and secretion of type I interferons. Antigen-presenting cells or APCs will be discussed in greater detail in Unit 6.
Macrophages
When monocytes leave the blood and enter the tissue, they become activated and differentiate into macrophages. Those that have recently left the blood during inflammation and move to the site of infection through positive chemotaxis are sometimes referred to as wandering macrophages. In addition, the body has macrophages already stationed throughout all tissues and organs of the body. These are sometimes referred to as fixed macrophages.
Many fixed macrophages are part of the mononuclear phagocytic (reticuloendothelial) system. They, along with B-lymphocytes and T-lymphocytes, are found supported by reticular fibers in lymph nodules, lymph nodes, and the spleen where they filter out and phagocytose foreign matter such as microbes. Similar cells are also found in the liver (Kupffer cells), the kidneys (mesangial cells), the brain (microglia), the bones (osteoclasts), the lungs (alveolar macrophages), and the gastrointestinal tract (peritoneal macrophages). Macrophages actually have a number of very important functions in body defense including:
Function 1
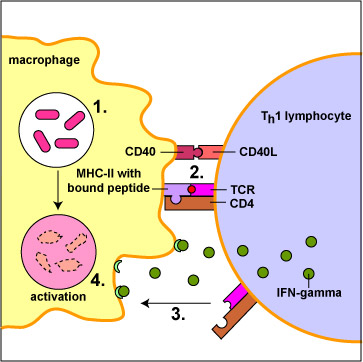
Killing of microbes, infected cells, and tumor cells by phagocytosis. Macrophages that have engulfed microorganisms become activated by a subset of T-helper lymphocytes called Th1 cells (Figure \(\PageIndex{6}\)). Activated macrophages develop a ruffled cytoplasmic membrane and produce increased numbers of lysosomes.
Function 2
Processing antigens so they can be recognized by effector T-lymphocytes during the adaptive immune responses. Macrophages, as well as the dendritic cells mentioned below, process antigens through phagocytosis and present them to T-lymphocytes. Because of this function, they are often referred to as antigen-presenting cells or APCs.
Macrophages primarily capture and present protein antigens to effector T-lymphocytes. (Effector lymphocytes are lymphocytes that have encountered an antigen, proliferated, and matured into a form capable of actively carrying out immune defenses.) Macrophages engulf the microorganism and degrade it with their lysosomes. Peptides from microbial proteins are then bound to a groove of unique molecules called MHC-II molecules produced by macrophages, dendritic cells, and B-lymphocytes. The peptide epitopes bound to the MHC-II molecules are then put on the surface of the macrophage (Figure \(\PageIndex{1}\)) where they can be recognized by complementary shaped T-cell receptors (TCR) and CD4 molecules on an effector T4-lymphocyte (Figure \(\PageIndex{2}\)). This interaction leads to the activation of that macrophage.
Like dendritic cells discussed above, macrophages are also capable of capturing and presenting protein antigens to naive T-lymphocytes although they are not as important in this function.
Function 3
Secreting lipid mediators of inflammation such as leukotrienes, prostaglandins, and platelet-activating factor (PAF).
Function 4
Secreting proteins called cytokines that play a variety of roles in non-specific body defense. Macrophage-produced cytokines promote inflammation and induce fever, increase phagocytosis and energy output, promote sleep, activate resting T-lymphocytes , attract and activate neutrophils, and stimulate the replication of endothelial cells to form capillaries and fibroblasts to form connective scar tissue. Four important cytokines that macrophages produce (as mentioned in Unit 1 under endotoxin) are tumor necrosis factor-alpha (TNF-alpha), interleukin-1 (IL-1), interleukin-6 (IL-6), and interleukin-8 (IL-8).
There is growing evidence that monocytes and macrophages can be “trained” by an earlier infection to do better in future infections, that is, develop memory. It is thought that microbial pathogen-associated molecular patterns (PAMPs) binding to pattern-recognition (PRRs) on monocytes and macrophages triggers the cell’s epigenome to reprogram or train that cell to react better against new infections.
Macrophages show great functional diversity. In addition to the populations of macrophages involved in body defense and immunity, there are populations of macrophages that play important roles in:
- The development of a variety of tissues and organs within the body, including the brain, blood cells, mammary gland, pancreas, and kidneys.
- Modulating normal physiology and maintaining homeostasis in the body, including insulin resistance and sensitivity, long term nutrient storage, thermogenesis, and liver and pancreas function in response to caloric intake.
- Tissue repair, including the formation of scar tissue and the growth of new capillaries into injured tissues.
Mast Cells
Mast cells are typically the immunological first responders to infection and carry out many of the same inflammatory-mediating functions as basophils. There are two types of mast cells in the body: mast cells found in the connective tissue and mast cells found throughout the mucous membranes. The granules of mast cells contain such mediators as histamine, eosinophil chemotactic factor, neutrophil chemotactic factor, platelet activating factor, and cytokines such as IL-3, IL-4, IL-5, IL-6, and TNF-alpha. They also possess pathways for synthesizing leukotrienes and prostaglandins, chemicals that promote inflammation by causing vasodilation, increasing capillary permeability, and increasing mucous production.
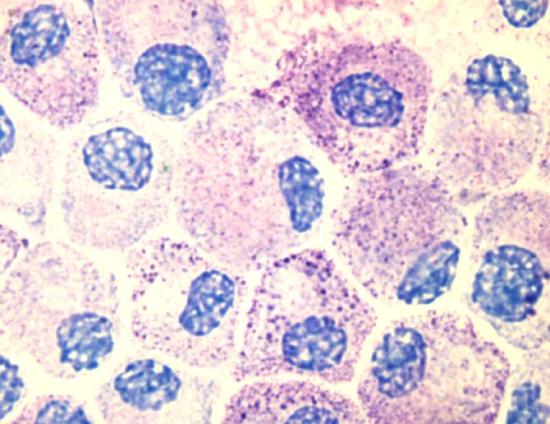
Photo of cultured mast cells at 100X using an oil immersion lens and an olympus digital camera. The cells are stained with Tol Blue, and might appear slightly degranulated as they were activated using an artificial antigen during the course of an experiment. Image use with permission (Kauczuk).
Mast cells have pattern-recognition receptors or PRRs on their surface that interact with pathogen-associated molecular patterns or PAMPs of microbes. After the PAMPs bind to their respective PRRs, they release the contents of their granules. These chemical mediators promote inflammation and attract neutrophils to the infected site.
Summary
- Most dendritic cells are derived from monocytes and are referred to as myeloid dendritic cells and are located throughout the epithelium of the skin, the respiratory tract, and the gastrointestinal tract, as well as lymphoid tissues and organ parenchyma.
- Upon capturing antigens through pinocytosis and, the dendritic cells detach from their initial site, enter lymph vessels, and are carried to regional lymph nodes where they present antigens to the ever changing populations of naive T-lymphocytes.
- The primary function of dendritic cells is to capture and present protein antigens to naive T-lymphocytes.
- When monocytes leave the blood and enter the tissue, many become activated and differentiate into macrophages. These macrophages that have recently left the blood during inflammation and move to the site of infection through positive chemotaxis are sometimes referred to as wandering macrophages.
- The body has macrophages already stationed throughout the tissues and organs of the body and these are sometimes referred to as fixed macrophages.
- Functions of macrophages include killing of microbes, infected cells, and tumor cells by phagocytosis, processing antigens so they can be recognized by effector T-lymphocytes during the adaptive immune responses, and secreting mediators of inflammation such as leukotrienes, prostaglandins, and platelet-activating factor, and cytokines.
- Mast cells are typically the immunological first responders to infection and carry out many of the same inflammatory-mediating functions as basophils.


