10.2: Testing for Mutagenic Chemicals in Bacteria and Mice
- Page ID
- 4897
Ames test
Big Blue mice are transgenic for a segment of DNA that contains the DNA of bacteriophage lambda, a virus that infects E. coli, and which serves here as the vector for 3 genetic elements from the lac operon of E. coli:
- the lacI gene
- the operator of the operon
- the beta-galactosidase (lacZ) gene
The Assay
The transgenic mice are given repeated doses of the suspected carcinogen for a week or two. If the chemical is mutagenic, it will cause random mutations throughout the genome of each mouse cell. If a mutation occurs in either the lacI gene (which encodes the lac repressor) or the operator,
the gene (lacZ) for beta-galactosidase will no longer be repressed. To detect this,
- The DNA is extracted from the tissues of the treated mouse.
- The vector is isolated and used to make functional bacteriophages.
- E. coli cells are mixed with the bacteriophage and spread on a solid culture medium.
- The bacteriophages infect and destroy ("lyze") the E. coli cells.
- This causes clear circular zones, called plaques, to appear in a "lawn" of bacteria.
- Before they die, cells that have been infected by bacteriophages carrying a mutated lacI or operator will produce beta-galactosidase.
- This reacts with a substrate in the culture medium turning it blue.
- Bacteriophages with unmutated genes produce colorless plaques because no beta-galactosidase is synthesized.
- Count both colorless and blue plaques.
- The number of blue plaques divided by the total number of plaques gives the mutation frequency.

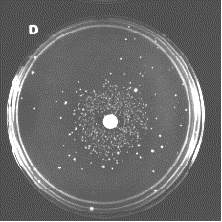
This photograph shows one mutant (blue) plaque on a lawn of E. coli containing many non-mutant (clear) plaques.


