W2018_Bis2A_Lecture06_reading
- Page ID
- 25318
Characteristic Chemical Reactions
All chemical reactions begin with a reactant—the general term for the one or more substances that enter into the reaction. Sodium and chloride ions, for example, are the reactants in the production of table salt. The one or more substances produced by a chemical reaction are called the product.
In chemical reactions, the atoms and elements present in the reactant(s) must all also be present in the product(s). Similarly, there can be nothing present in the products that was not present in the reactants. This is because chemical reactions are governed by the law of conservation of mass, which states that matter cannot be created nor destroyed in a chemical reaction. This means that when you examine a chemical reaction, you must try to account for everything that goes in AND make sure that you can find it all in the stuff that comes out!
Just as you can express mathematical calculations in equations such as 2 + 7 = 9, you can use chemical equations to show how reactants become products. By convention, chemical equations are typically read or written from left to right. Reactants on the left are separated from products on the right by a single- or double-headed arrow indicating the direction in which the chemical reaction proceeds. For example, the chemical reaction in which one atom of nitrogen and three atoms of hydrogen produce ammonia would be written as:
\[N + 3H→NH_3.\]
Correspondingly, the breakdown of ammonia into its components would be written as:
\[NH3→N + 3H.\]
Note that in either direction, you find 1 N and 3 Hs on both sides of the equation.
Reversibility
In theory, any chemical reaction can proceed in either direction under the right conditions. Reactants may synthesize into a product that later reverts back to a reactant. Reversibility is also a quality of exchange reactions. For instance, A+BC→AB+C could then reverse to AB+C→A+BC. This reversibility of a chemical reaction is indicated with a double arrow: A+BC⇄AB+C.
Synthesis reactions
Many macromolecules are made from smaller subunits, or building blocks, called monomers. Monomers covalently link to form larger molecules known as polymers. Often, the synthesis of polymers from monomers will also produce water molecules as products of the reaction. This type of reaction is known as dehydration synthesis or condensation reaction.
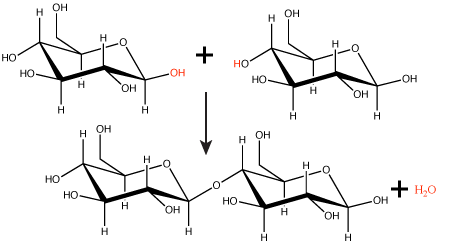
Figure 1. In the dehydration synthesis reaction depicted above, two molecules of glucose are linked together to form the disaccharide maltose. In the process, a water molecule is formed.
Attribution: Marc T. Facciotti (original work)
In a dehydration synthesis reaction (Figure 1), the hydrogen of one monomer combines with the hydroxyl group of another monomer, releasing a molecule of water. At the same time, the monomers share electrons and form covalent bonds. As additional monomers join, this chain of repeating monomers forms a polymer. Different types of monomers can combine in many configurations, giving rise to a diverse group of macromolecules. Even one kind of monomer can combine in a variety of ways to form several different polymers; for example, glucose monomers are the constituents of starch, glycogen, and cellulose.
In the carbohydrate monomer example above, the polymer is formed by a dehydration reaction; this type of reaction is also used to add amino acids to a growing peptide chain and nucleotides to the growing DNA or RNA polymer. Visit the modules on Amino Acids, Lipids, and Nucleic Acids to see if you can identify the water molecules that are removed when a monomer is added to the growing polymer.
![]()
Figure 2. This depicts, using words, (decorated with functional groups colored in red) a generic dehydration synthesis/condensation reaction.
Attribution: Marc T. Facciotti (original work)
Hydrolysis reactions
Polymers are broken down into monomers in a reaction known as hydrolysis. A hydrolysis reaction includes a water molecule as a reactant (Figure 3). During these reactions, a polymer can be broken into two components: one product carries a hydrogen ion (H+) from the water, while the second product carries the water's remaining hydroxide (OH–).
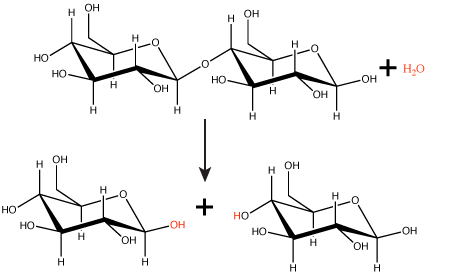
Figure 3. In the hydrolysis reaction shown here, the disaccharide maltose is broken down to form two glucose monomers with the addition of a water molecule. Note that this reaction is the reverse of the synthesis reaction shown in Figure 1 above.
Attribution: Marc T. Facciotti (original work)
![]()
Figure 4. This depicts using words (decorated with functional groups colored in red) a generic hydrolysis reaction.
Attribution: Marc T. Facciotti (original work)
Dehydration synthesis and hydrolysis reactions are catalyzed, or “sped up,” by specific enzymes. Note that both dehydration synthesis and hydrolysis reactions involve the making and breaking of bonds between the reactants—a reorganization of the bonds between the atoms in the reactants. In biological systems (our bodies included), food in the form of molecular polymers is hydrolyzed into smaller molecules by water via enzyme-catalyzed reactions in the digestive system. This allows for the smaller nutrients to be absorbed and reused for a variety of purposes. In the cell, monomers derived from food may then be reassembled into larger polymers that serve new functions.
Helpful links:
Visit this site to see visual representations of dehydration synthesis and hydrolysis.
Example of Hydrolysis with Enzyme Action is shown in this 3 minute video entitled: Hydrolysis of Sucrose by Sucrase.
Exchange/transfer reactions
We will also encounter reactions termed exchange reactions. In these types of reactions, "parts" of molecules are transferred between one another—bonds are broken to release a part of a molecule and bonds are formed between the released part and another molecule. These enzyme-catalyzed reactions are usually reasonably complex multistep chemical processes.

Figure 5. An exchange reaction in which both synthesis and hydrolysis can occur, chemical bonds are both formed and broken, is depicted using a word analogy.
The energy story
Overview of the energy story
Whether we know it or not, we tell stories that involve matter and energy everyday. We just don’t often use terminology associated with scientific discussions of matter and energy.
Example 1
The setup: a simple statement with implicit details
You tell your roommate a story about how you got to campus by saying, "I biked to campus today." In this simple statement are several assumptions that are instructive to unpack, even if they may not seem very critical to include explicitly in a casual conversation between friends over transportation choices.
An outsider's reinterpretation of the process
To illustrate this, imagine an external observer, such as an alien being watching the comings and goings of humans on Earth. Without the benefit of knowing much of the implied meanings and reasonable assumptions that are buried in our language, the alien's description of the morning cycling trip would be quite different than your own. What you described efficiently as "biking to campus" might be more specifically described by the alien as a change in location of a human body and its bicycle from one location (the apartment, termed position A) to a different location (the university, termed position B). The alien might be even more abstract and describe the bike trip as the movement of matter (the human body and its bike) between an initial state (at location A) to a final state (at location B). Furthermore, from the alien's standpoint, what you'd call "biking" might be more specifically described as the use of a two-wheeled tool that couples the transfer of energy from the electric fields in chemical compounds to the acceleration of the two-wheeled, tool-person combo that heats its environment. Finally, buried within the simple statement describing how we got to work is also the tacit understanding that the mass of the body and bike were conserved in the process (with some important caveats we’ll look at in future lectures) and that some energy was converted to enable the movement of the body from position A to position B.
Note: possible discussion
Details are important. What if you owned a fully electric bike, and the person you were talking with didn’t know that? What important details might this change about the “everyday” story you told that the more detailed description would have cleared up? How would the alien’s story have changed? In what scenarios might these changes be relevant?
As this simple story illustrates, irrespective of many factors, the act of creating a full description of a process includes some accounting of what happened to the matter, what happened to the energy, and almost always some description of a mechanism that describes how changes in matter and energy of a system were brought about.
To practice this skill in BIS2A, we will make use of something we like to call the "Energy Story." You may be asked to tell an "energy story" in class, to practice telling energy stories on your lecture study guides, and to use the concept on your exams. In this section, we focus primarily on introducing the concept of an energy story and explaining how to tell one. It is worth noting that the term "energy story" is used almost exclusively in BIS2A (and has a specific meaning in this class). This precise term will not appear in other courses at UC Davis (at least in the short term), or if it appears, is not likely to be used in the same manner. You can think of “The Energy Story” as a systematic approach to creating a statement or story describing a biological process or event. Your BIS2A instructors have given this approach a short name (energy story) so that we can all associate it with the common exercise. That way, when the instructor asks the class to tell or construct an energy story, everyone knows what is meant.
Definition 1: energy story
An energy story is a narrative describing a process or event. The critical elements of this narrative are as follows:
- Identify at least two states (e.g., start and end) in the process.
- Identify and list the matter in the system and its state at the start and end of the process.
- Describe the transformation of the matter that occurs during the process.
- Account for the “location” of energy in the system at the start and end of the process.
- Describe the transfer of energy that happens during the process.
- Identify and describe mechanism(s) responsible for mediating the transformation of matter and transfer of energy.
A complete energy story will include a description of the initial reactants and their energetic states as well as a description of the final products and their energetic states after the process or reaction is completed.
Note: possible discussion
We argue that the energy story can be used to communicate all of the useful details that are required to describe nearly any process. Can you think of a process that cannot be adequately described by an energy story? If so, describe such a process.
Example 2: energy story example
Let us suppose that we are talking about the process of driving a car from "Point A" to "Point B" (see Figure 1).
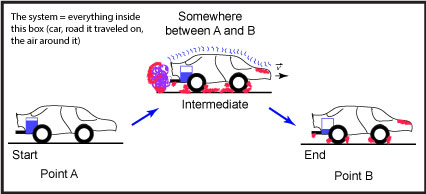
Figure 1: This is a schematic of a car moving from a starting position, "Point A," to an end point, "Point B." The blue rectangle depicted in the back of the car represents the level of the gasoline; the purple, squiggly line near the exhaust pipe represents the exhaust; squiggly blue lines on top of the car represent sound vibrations; and the red shading represents areas that are hotter than at the start. Source: created by Marc T. Facciotti (own work)
Let's step through the Energy Story rubric:
1. Identify at least two states (e.g., start and end) in the process.
In this example, we can easily identify two states. The first state is the nonmoving car at "Point A," the start of the trip. The second state, after the process is done, is the nonmoving car at "Point B."
2. Identify and list the matter in the system and its state at the start and end of the process.
In this case, we first note that the "system" includes everything in the figure—the car, the road, the air around the car, etc.
It is important to understand the we are going to apply the physical law of conservation of matter. That is, in any of the processes that we will discuss, matter is neither created or destroyed. It might change form, but one should be able to account for everything at the end of a process that was there at the beginning.
At the beginning of the process, the matter in the system consists of the following:
1. The car and all the stuff in it
2. The fuel in the car (a special thing in the car)
3. The air (including oxygen) around the car.
4. The road
5. The driver
At the end of the process, the matter in the system is distributed as follows:
1. The car and all the stuff in it is in a new place (let's assume, aside from the fuel and position, that nothing else changed).
2. There is less fuel in the car, and it too is in a new place.
3. The air has changed; it now has less molecular oxygen, more carbon dioxide, and more water vapor.
4. The road did not change (let's assume it didn't change—other than a few pebbles that moved around).
5. The driver did not change (let's assume she didn't change—though we'll see by the end of the term that she did, at least a little). However, the driver is now in a different place.
3. Describe the transformation of the matter that occurs during the process.
What happened to the matter in this process? Thanks to a lot of simplifying assumptions, we see that two big things happened. First, the car and its driver changed positions—they went from "Point A" to "Point B." Second, we note that some of the molecules in the fuel, which used to be in the car as a liquid, have changed forms and are now mostly in the form of carbon dioxide and water vapor (purple blob coming out of the tailpipe). Some of the oxygen molecules that used to be in the air are now also in a new place as part of the carbon dioxide and water that left the car.
4. Account for the “location” of energy in the system at the start and end of the process.
It is again important to understand that we are going to invoke the physical law of conservation of energy. That is, we stipulate that the energy in the system cannot be created or destroyed, and therefore, the energy that is in the system at the start of the process must still be there at the end of the process. It may have been redistributed, but you should be able to account for all the energy.
At the beginning of the process, the energy in the system is distributed as follows:
1. The energy is tied up in the associations between atoms that make up the matter of the car.
2. The energy is tied up in the associations between atoms that make up the fuel.
3. The energy is tied up in the associations between atoms that make up the air.
4. The energy is tied up in the associations between atoms that make up the road.
5. The energy is tied up in the associations between atoms that make up the driver.
6. For all the things above, we can also say that there is energy in the molecular motions of the atoms that make up the stuff.
At the end of the process, the energy in the system is distributed as follows:
1. The energy is tied up in the associations between atoms that make up the matter of the car.
2. The energy is tied up in the associations between atoms that make up the fuel.
3. The energy is tied up in the associations between atoms that make up the air.
4. The energy is tied up in the associations between atoms that make up the road.
5. The energy is tied up in the associations between atoms that make up the driver.
6. For all the things above, we can also say that there is energy in the molecular motions of the atoms that make up the stuff.
This is interesting in some sense, because the lists are about the same. We know that the amount of energy stored in the car has decreased, because there is less fuel. Something must have happened.
5. Describe the transfer of energy that happens during the process.
In this particular example, it is the transfer of energy among the components of the system that is most interesting. As we mentioned, there is less energy stored in the gas tank of the car at the end of the trip, because there is now less fuel. We also know intuitively (from real-life experience) that the transfer of energy from the fuel to something else was instrumental in moving the car from "Point A" to "Point B." So, where did this energy go? Remember, it didn't just disappear. It must have moved somewhere else in the system.
Well, we know that there is more carbon dioxide and water vapor in the system after the process. There is energy in the associations between those atoms (atoms that used to be in the fuel and air). So some of the energy that was in the fuel is now in the exhaust. Let's also draw from real-life experience again, and state that we know that parts of our car have gotten hot by the end of the trip (e.g., the engine, transmission, wheels/tires, exhaust, etc.). For the moment, we'll just use our intuition, and say that we understand that making something hot involves some transfer of energy. So we can reasonably postulate that some of the energy in the fuel went (directly or indirectly) into heating the car, parts of the road, and the exhaust—and thus the environment around the car. An amount of energy also went into accelerating the car from zero velocity to whatever speed it traveled at, but most of that energy eventually became heat when the car came to a stop.
This is a bit of a hand-wavy explanation, and we'll learn how to do a better job throughout the quarter. The main point is that we should be able to add all the energy of the system at the beginning of the process (in all the places it is found) and at the end of the process (in all the places it is found), and those two values should be the same.
6. Identify and describe mechanism(s) responsible for mediating the transformation of matter and transfer of energy.
Finally, it is useful to try understanding how those transformations of matter and transfers of energy might have been facilitated. For the sake of brevity, we might just say that there was a complicated mechanical device (the engine) that helped facilitate the conversion of matter and transfer of energy about the system and coupled this to the change in position of the car. Someone interested in engines would, of course, give a more detailed explanation.
In this example, we made a bunch of simplifying assumptions to highlight the process and to focus on the transformation of the fuel. But that's fine. The more you understand about the processes, the finer details you can add. Note that you can use the Energy Story rubric for describing your understanding (or looking for holes in your understanding) of nearly any process (certainly in biology). In BIS2A, we'll use the Energy Story to get an understanding of processes as varied as biochemical reactions, DNA replication, the function of molecular motors, etc.
Important:
First: We will be working on many examples of the energy story throughout the course—do not feel that you need to have mastery over this topic today.
Second: While it is tempting to think all this is superfluous or not germane to your study of biology in BIS2A, let this serve as a reminder that your instructors (those creating the course midterm and final assessments) view it as core material. We will revisit this topic often throughout the course but need you to get familiar with some of the basic concepts now.
This is important material and an important skill to develop—do not put off studying it because it doesn't "look" like "biology" to you today. The academic term moves VERY quickly, and it will be difficult to catch up later if you don't give this some thought now.

