Investigation: Cellular Respiration
This page is a draft and is under active development.
( \newcommand{\kernel}{\mathrm{null}\,}\)
What Factors Affect Cellular Respiration?
 This investigation uses respirometry techniques to calculate the rate of oxygen consumption (cellular respiration) in germinating pea seeds. The effect of temperature and whether a seed has broken dormancy are quantified and graphed. The ideal gas law and its concepts are reviewed and applied.
This investigation uses respirometry techniques to calculate the rate of oxygen consumption (cellular respiration) in germinating pea seeds. The effect of temperature and whether a seed has broken dormancy are quantified and graphed. The ideal gas law and its concepts are reviewed and applied.
Basic Questions (scientific practices)
● What is the relationship between temperature, volume and pressure?
● How can respiration rates be measured using a respirometer?
Experimental Questions:
● How is respiration rate affected by temperature?
● How does the respiration rate of a germinating seed differ from that of a dormant seed?
Background
Each individual cell is responsible for the energy exchanges necessary to sustain its ordered structure. Cells accomplish this task by breaking down nutrient molecules to generate ATP (adenosine triphosphate), which can then be used to run cellular processes that require energy. This process is called cellular respiration which requires nutrient molecules and oxygen. Carbon dioxide and water are products of the series of reactions involved in cellular respiration.
C6H12O6 + 6CO2 → 6CO2 + 6H2O
Measuring the rate of cellular respiration can either rely on measuring the amount of oxygen taken in, or the amount of carbon dioxide being released. Respirometers are devices that measure these types of gas volume changes, and therefore provide information about the rate of cellular respiration.
The ideal gas law, describes the relationship between temperature, pressure and volume. (PV = nrT)
During cellular respiration, two gases are changing in volume. Oxygen gas is being consumed by the respiring cells and carbon dioxide gas is diffusing out of the cells. The respirometer, therefore, has to be able to deal with two simultaneously changing gas volumes. This is accomplished by introducing potassium hydroxide into the device. KOH absorbs carbon dioxide, following this equation
CO2 + 2KOH → K2CO3 + H2O
Potassium carbonate (K2CO3) is a solid precipitate. Any CO2 produced is immediately converted from a gas to a solid and is therefore no longer governed by gas laws. This allows the respirometer to measure only one variable, the consumption of oxygen gas by living cells.
Assembling the Respirometers
Two sets of respirometers will be assembled during this lab exercise. Each set will be incubated at a different temperature. One respirometer will contain germinating seeds, one will contain a mix of nongerminating seeds and plastic beads with a volume equal to the first vial. Respirometers will be submerged in a pan of either cold or room temperature water and the rate of respiration will be measured by observing the movement of water into the pipet.
Lab Materials: Germinating pea seeds, dry pea seeds, plastic beads, 2 respirometers, absorbent cotton, nonabsorbent cotton, 1 round wood stick, water bath, ice, 100 ml graduated cylinder, stopwatch or clock, water. Dropper Bottle of 15% KOH Safety: Wear safety goggles. KOH is caustic, avoid direct skin contact.
Procedure:
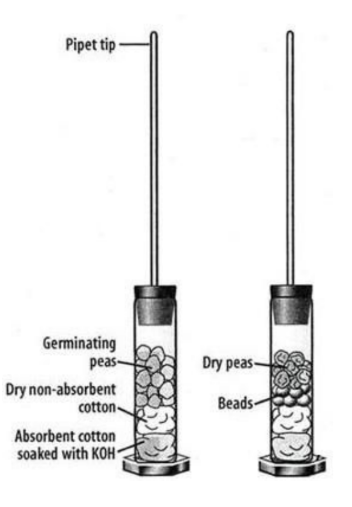 1. Fill a graduated cylinder with 20 ml of water. Place 20 germinating seeds in the cylinder and record the volume by reading the new level of water. Remove seeds and place on a paper towel. Volume of germinating seeds: ______
1. Fill a graduated cylinder with 20 ml of water. Place 20 germinating seeds in the cylinder and record the volume by reading the new level of water. Remove seeds and place on a paper towel. Volume of germinating seeds: ______
2. Fill a graduated cylinder with 20 ml of water. Place 20 non germinating seeds in the water. Drop plastic beads into the cylinder until the volume is the same as the germinating seeds from step 1.
3. Obtain two glass respirometer vials. Place absorbent cotton in the bottom and add KOH to saturate the cotton. It should be soaked, but no dripping. Caution: KOH will burn the skin!
4. Place nonabsorbent cotton over the KOH soaked absorbent cotton to act as a barrier so your samples do not come into contact with the caustic substance.
5. Add the peas, peas/beads, and beads to the appropriate respirometer. Place the stoppers on each of the vials and ensure they are secured tightly. You will be assigned a temperature to measure the respiration rates (cold or room temperature).
6. Create your water bath with your assigned temperature. Lean your respirometers on the edge of the bath so that the temperature inside the chamber equals the temperature of the bath. Do not submerge them yet! Let them equalize for about 5 minutes.
7. If your water bath is in a dark plastic bin, place white paper towels at the bottom to make it easier to read the pipet.
8. Submerge your respirometers, a little bit of water will enter the tips at first. You may need to rotate the pipet in order to take readings, but once you have them submerged and situated, limit movement as this will affect your results.
9. If your respirometers float, you may need to weight them. You can improvise here, stainless steel dissection scissors; for instance, can serve to weight the tubes.
10. Collect data on the data table for your assigned temperature. You will take readings at 4-minute intervals.

11. Note: Read the pipet as the water bubble moves down the tube toward the respirometers. You are using a 1 ml pipet, so each of the units on the reading are .9, .8, .7, etc. The reading shown on the above image would be .74
*If you are having trouble finding the bubble, place a drop of food coloring in the water near the tip of the pipet.
DATA & ANALYSIS
Collect data on your assigned temperature. O2 consumed is measured by subtracting the initial pipet reading from the original reading. Example. If pipet started at .8 and then at 4 minutes it was .7, O2 consumption = .1
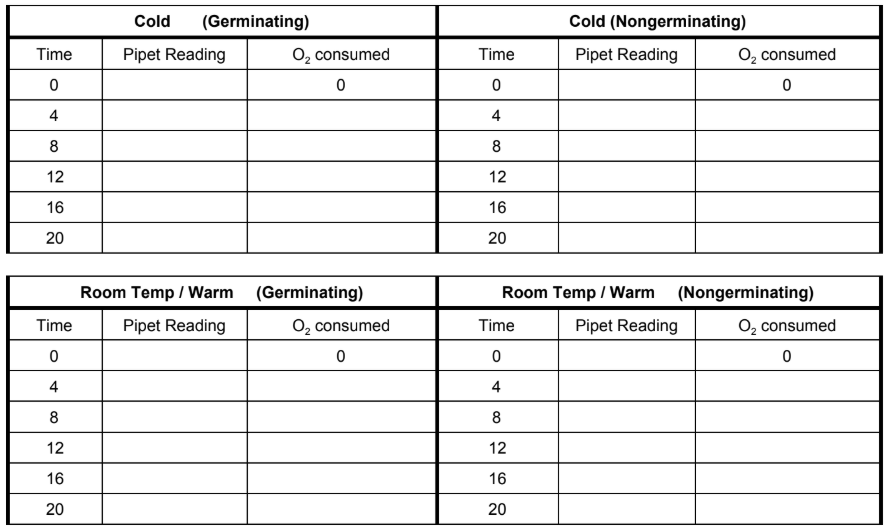
Graph: Graph your data below. There will be 4 lines on this graph; label and color code. Draw a line of best fit.

Calculate Slope The slope of your lines will represent the respiration rate of each of your samples:
Respiration Rates for each sample:
Analysis
1. State a hypothesis that relates to temperature that is being tested by this lab exercise.
2. State a hypothesis that relates to the state of seed germination that is being tested by this lab exercise.
3. In this lab exercise, what is the purpose of the ....
- Beads
- KOH
- Respirometer
4. Explain why it was important that the vials contained the same volume. Use the Ideal Gas Law in your explanation.
5. The instructor that created this lab did not have you make a control, though many other similar labs require students to assemble a third respirometer as a control. Review how you designed your tests and suggest what your control respirometer would look like. It will not just be an empty vial.

