18.7.3: Procedure Antibiotic Susceptibility Testing
- Page ID
- 122776
Videos reviewing techniques used in this lab:
MATERIALS
- 150mm Mueller-Hinton agar plates (3)
- Sterile swabs (3)
- An antibiotic disc dispenser containing discs of antibiotics commonly effective against Gram-positive bacteria, and one containing discs of antibiotics commonly effective against Gram-negative bacteria
ORGANISMS
Trypticase Soy broth cultures of Staphylococcus aureus (Gram-positive), Enterococcus faecalis (Gram-positive), and Pseudomonas aeruginosa (Gram-negative)
ANTIBIOTIC SUSCEPTIBILITY TESTING PROCEDURE: to be done in groups of 3
The basic steps for the Bauer-Kirby method of antimicrobial susceptibility testing are given below. This outline of procedure is intended to be used as an adjunct to general microbiology laboratory instruction. The procedure is highly regulated and controlled by the Clinical and Laboratory Standards Institute (CLSI) and must be accompanied by a rigorous quality assurance program including performance by certified and/or licensed personnel when the results are to be reported in clinical settings .
1. Take 3 Mueller-Hinton agar plates. Label one S. aureus, one E. faecalis, and one P. aeruginosa.
2. Using your wax marker, divide each plate into thirds to guide your streaking.
|
|
3. Dip a sterile swab into the previously-standardized tube of S. aureus. Squeeze the swab against the inner wall of the tube to remove excess liquid.
4. Streak the swab perpendicular to each of the 3 lines drawn on the plate overlapping the streaks to assure complete coverage of the entire agar surface with inoculum.
|
|
5. Repeat steps 3 and 4 for the E. faecalis and P. aeruginosa plates.
6. Using the appropriate antibiotic disc dispenser, place Gram-positive antibiotic-containing discs on the plates of S. aureus and E. faecalis; Gram-negative antibiotic-containing discs on the plate of P. aeruginosa.
7. Incubate the 3 plates upside down and stacked on the shelf of the 35°C incubator corresponding to your lab section until the next lab period.
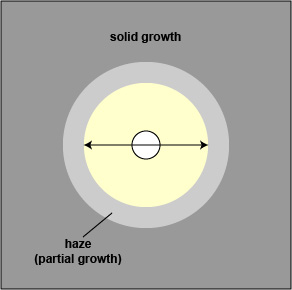
8 . Using a metric ruler, measure the diameter of the zone of inhibition around each disc on each plate in mm by placing the ruler on the bottom of the plate (Fig. \(\PageIndex{1}\)).
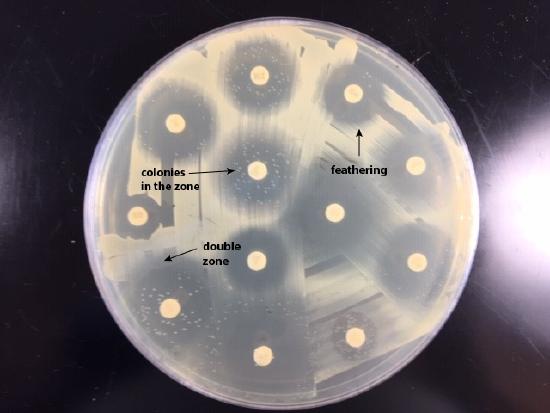
- If there is a double zone of inhibition, measure the diameter of the innermost zone (see Fig. \(\PageIndex{2A}\) and Fig. \(\PageIndex{2B}\)).
|
Fig. \(\PageIndex{2A}\): |
Fig. \(\PageIndex{2B}\): |
|---|---|
|
|
| If there is a double zone of inhibition, measure the diameter of the innermost zone (arrowed line). | Examples of double zone of inhibition, colonies in the zone, and feathering. |
| Copyright; Gary E. Kaiser, Ph.D. The Community College of Baltimore County, Catonsville Campus CC-BY-3.0 | |
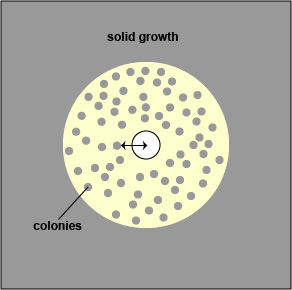
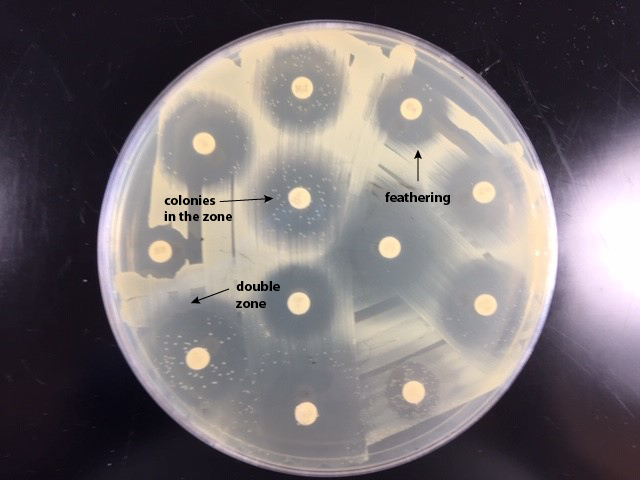
If there is a zone containing colonies, measure the diameter of the colony free zone. Measure from the colony closest to the antibiotic disc to the center of the disc (the radius) and double that number to get the diameter. (See Fig. \(\PageIndex{3A}\) and Fig. \(\PageIndex{3B}\)).
|
Fig. \(\PageIndex{3A}\): |
Fig. \(\PageIndex{3A}\): |
|---|---|
|
|
| If there is a zone containing colonies, measure the diameter of the colony free zone (arrowed line). | Examples of double zone of inhibition, colonies in the zone, and feathering. |
|
Copyright; Gary E. Kaiser, Ph.D. The Community College of Baltimore County, Catonsville Campus CC-BY-3.0 |
|
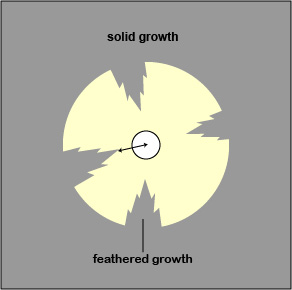
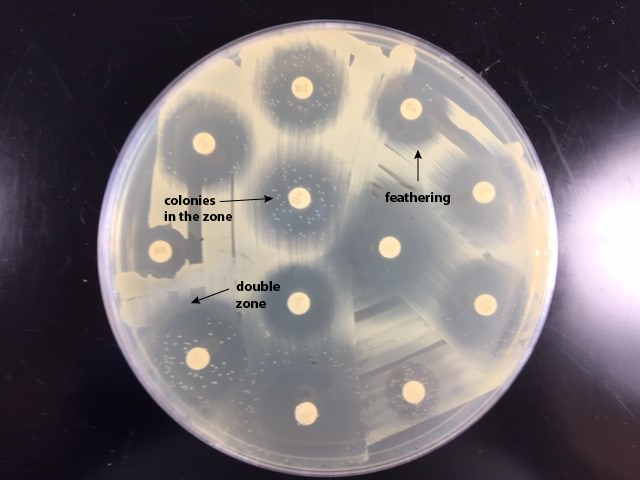
- If there is a feathered zone, measure the diameter of the point where there is an obvious demarcation between growth and no growth. Measure from the end of the feathering closest to the antibiotic disc to the center of the disc (the radius) and double that number to get the diameter. (See Fig. \(\PageIndex{4A}\) and Fig. \(\PageIndex{4B}\)).
|
Fig. \(\PageIndex{4A}\): |
Fig. \(\PageIndex{4A}\): |
|---|---|
|
|
| If there is a zone containing feathered growth, measure the diameter of the growth-free zone (arrowed line). | Examples of double zone of inhibition, colonies in the zone, and feathering. |
| Copyright; Gary E. Kaiser, Ph.D. The Community College of Baltimore County, Catonsville Campus CC-BY-3.0 | |
- If there is a double zone of inhibition, measure the diameter of the innermost zone (see Fig. \(\PageIndex{2A}\) and Fig. \(\PageIndex{2B}\)).
- If there is a zone containing colonies, measure the diameter of the colony free zone. Measure from the colony closest to the antibiotic disc to the center of the disc (the radius) and double that number to get the diameter. (See Fig. \(\PageIndex{3A}\) and Fig. \(\PageIndex{3B}\).).
- If there is a feathered zone, measure the diameter of the point where there is an obvious demarcation between growth and no growth. Measure from the end of the feathering closest to the antibiotic disc to the center of the disc (the radius) and double that number to get the diameter. (See Fig. \(\PageIndex{4A}\) and Fig. \(\PageIndex{4B}\)).
- When testing Staphylococcus aureus, the haze around an oxacillin should not be ignored. Measure the diameter of the zone free of growth or haze.
9 . Determine whether each organism is susceptible, moderately susceptible, intermediate, or resistant to each chemotherapeutic agent using the standardized table (Table 2) and record your results.
Table \(\PageIndex{1a}\) Zone Size Interpretive Chart for Bauer-Kirby Test| Antimicrobial agent | Disc code | R = mm or less | I = mm | MS = mm | S = mm or more |
|---|---|---|---|---|---|
| amikacin |
AK-30 |
15 |
15-16 |
- |
16 |
| amoxicillin/ clavulanic acid - Staphylococcus |
AMC-30 |
19 |
- |
- |
20 |
| amoxicillin/ clavulanic acid - other organisms |
AMC-30 |
13 |
14-17 |
- |
18 |
| ampicillin - Staphylococcus |
AM-10 |
28 |
- |
- |
29 |
| ampicillin - G- enterics |
AM-10 |
11 |
12-13 |
- |
14 |
| Antimicrobial agent | Disc code | R = mm or less | I = mm | MS = mm | S = mm or more |
|---|---|---|---|---|---|
| azlocillin |
AZ-75 |
14 |
15-17 |
- |
13 |
| aztreonam |
ATM-30 |
15 |
- |
16-21 |
22 |
| carbenicillin - Enterobacteriaceae |
CB-100 |
17 |
18-22 |
- |
23 |
| carbenicillin - Pseudomonas |
CB-100 |
13 |
14-16 |
- |
17 |
| cefamandole |
MA-30 |
14 |
15-17 |
- |
18 |
| cefazolin |
CZ-30 |
14 |
15-17 |
- |
18 |
Table \(\PageIndex{1b}\) Zone Size Interpretive Chart for Bauer-Kirby Test
| Antimicrobial agent | Disc code | R = mm or less | I = mm | MS = mm | S = mm or more |
|---|---|---|---|---|---|
| cefonicid | CID-30 | 14 | 15-17 | - | 18 |
| cefoperazone | CFP-75 | 15 | - | 16-20 | 21 |
| cefotaxime | CTX-30 | 14 | - | 15-22 | 23 |
| cefotetan | CTT-30 | 12 | - | 13-15 | 16 |
| cefoxitin | FOX-30 | 13 | - | 15-17 | 18 |
| ceftazidime | CAZ-30 | 14 | 15-17 | - | 18 |
Table \(\PageIndex{1c}\) Zone Size Interpretive Chart for Bauer-Kirby Test
| Antimicrobial agent | Disc code | R = mm or less | I = mm | MS = mm | S = mm or more |
|---|---|---|---|---|---|
| ceftizoxime - Pseudomonas |
ZOX-30 |
10 |
- |
11 |
- |
| ceftizoxime - other organisms |
ZOX-30 |
14 |
- |
15-19 |
20 |
| ceftriaxone |
CRO-30 |
13 |
- |
14-20 |
21 |
| cefuroxime |
CXM-30 |
14 |
15-17 |
- |
18 |
| cephalothin |
CF-30 |
14 |
15-17 |
- |
18 |
| chloramphenicol |
C-30 |
12 |
13-17 |
- |
18 |
| cinoxacin |
CIN-100 |
14 |
15-18 |
- |
19 |
Table \(\PageIndex{1d}\) Zone Size Interpretive Chart for Bauer-Kirby Test
| Antimicrobial agent | Disc code | R = mm or less | I = mm | MS = mm | S = mm or more |
|---|---|---|---|---|---|
| ciprofloxacin |
CIP-5 |
15 |
16-20 |
- |
21 |
| clindamycin |
DA-2 |
14 |
15-20 |
- |
21 |
| doxycycline |
D-30 |
12 |
13-15 |
- |
16 |
| erythromycin |
E-15 |
13 |
14-22 |
- |
23 |
| gentamicin |
CN-10 |
12 |
13-14 |
- |
15 |
| imipenem |
IPM-10 |
13 |
14-5 |
- |
16 |
| kanamycin |
K-30 |
13 |
14-17 |
- |
18 |
Table \(\PageIndex{1e}\) Zone Size Interpretive Chart for Bauer-Kirby Test
| Antimicrobial agent | Disc code | R = mm or less | I = mm | MS = mm | S = mm or more |
|---|---|---|---|---|---|
| methicillin - Staphylococcus |
DP-5 |
9 |
10-13 |
- |
14 |
| mezlocillin |
MEZ-75 |
12 |
13-15 |
- |
16 |
| minocycline |
MI-30 |
14 |
15-18 |
- |
19 |
| moxalactam |
MOX-30 |
14 |
- |
15-22 |
23 |
| nafcillin - Staphylococcus |
NF-1 |
10 |
11-12 |
- |
13 |
| nalidixic acid |
NA-30 |
13 |
14-18 |
- |
19 |
| netilmicin |
NET-30 |
12 |
13-14 |
- |
17 |
Table \(\PageIndex{1f}\) Zone Size Interpretive Chart for Bauer-Kirby Test
| Antimicrobial agent | Disc code | R = mm or less | I = mm | MS = mm | S = mm or more |
|---|---|---|---|---|---|
| nitrofurantoin |
F/M-300 |
14 |
15-16 |
- |
17 |
| norfloxacin |
NOR-10 |
12 |
13-16 |
- |
17 |
| oxacillin - Staphylococcus |
OX-1 |
10 |
11-12 |
- |
13 |
| penicillin |
P-10 |
28 |
- |
- |
29 |
|
Piperacillin/Tazobactum |
TZP-110 |
17 |
18-20 |
- |
21 |
| sulfamethoxazole + trimethoprim |
SXT-25 |
10 |
11-15 |
- |
16 |
Table \(\PageIndex{1g}\) Zone Size Interpretive Chart for Bauer-Kirby Test
| Antimicrobial agent | Disc code | R = mm or less | I = mm | MS = mm | S = mm or more |
|---|---|---|---|---|---|
| tetracycline |
TE-30 |
14 |
15-18 |
- |
19 |
| ticarcillin |
TIC-75 |
11 |
12-14 |
- |
15 |
| ticarcillin/clavulanic acid |
TIM-85 |
11 |
12-14 |
- |
15 |
| tobramycin |
NN-10 |
12 |
13-14 |
- |
15 |
| trimethoprim |
TMP-5 |
10 |
11-15 |
- |
16 |
| vancomycin |
VA-30 |
9 |
10-11 |
- |
12 |
Contributors and Attributions
Dr. Gary Kaiser (COMMUNITY COLLEGE OF BALTIMORE COUNTY, CATONSVILLE CAMPUS)