17.4: Osmotic Pressure
- Page ID
- 122761
Microorganisms, in their natural environments, are constantly faced with alterations in osmotic pressure. Water tends to flow through semipermeable membranes, such as the cytoplasmic membrane of microorganisms, towards the side with a higher concentration of dissolved materials (solute). In other words, water moves from greater water (lower solute) concentration to lesser water (greater solute) concentration.
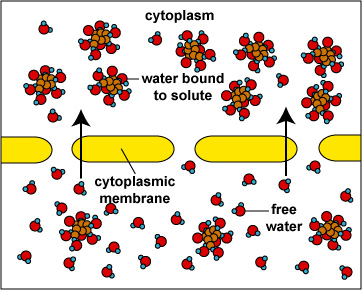
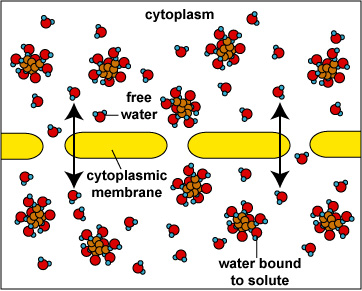
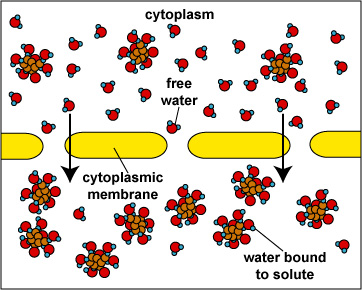
When the concentration of dissolved materials or solute is higher inside the cell than it is outside, the cell is said to be in a hypotonic environment and water will flow into the cell (Fig. 1). The rigid cell walls of bacteria and fungi, however, prevent bursting or plasmoptysis. If the concentration of solute is the same both inside and outside the cell, the cell is said to be in an isotonic environment (Fig. 2). Water flows equally in and out of the cell. Hypotonic and isotonic environments are not usually harmful to microorganisms. However, if the concentration of dissolved materials or solute is higher outside of the cell than inside, then the cell is in a hypertonic environment (Fig. 3). Under this condition, water flows out of the cell, resulting in shrinkage of the cytoplasmic membrane or plasmolysis. Under such conditions, the cell becomes dehydrated and its growth is inhibited.
|
Fig. \(\PageIndex{1}\) : |
Fig. \(\PageIndex{2}\): |
Fig. \(\PageIndex{3}\): |
|---|---|---|
|
|
|
| Copyright; Gary E. Kaiser, Ph.D. The Community College of Baltimore County, Catonsville Campus CC-BY-3.0 | ||
The canning of jams or preserves with a high sugar concentration inhibits bacterial growth through hypertonicity. The same effect is obtained by salt-curing meats or placing foods in a salt brine. This static action of osmotic pressure thus prevents bacterial decomposition of the food. Molds, on the other hand, are more tolerant of hypertonicity. Foods, such as those mentioned above, tend to become overgrown with molds unless they are first sealed to exclude oxygen. (Molds are aerobic.)
For more information on osmosis and the cytoplasmic membrane, see the following CourseArc lesson:
Contributors and Attributions
Dr. Gary Kaiser (COMMUNITY COLLEGE OF BALTIMORE COUNTY, CATONSVILLE CAMPUS)


