16.3.5: Detecting Antibody Using the Indirect Fluorescent Antibody Technique- The FTA-ABS test for syphilis
- Page ID
- 122741
The indirect fluorescent antibody technique involves three different reagents:
a. The patient's serum (containing antibodies against the disease antigen if the disease is present)
b. Known antigen for the suspected disease
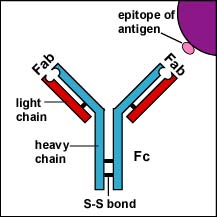
c. Fluorescent anti-human gamma globulin antibodies (antibodies made in another animal against the Fc portion of human antibodies (see Fig. \(\PageIndex{1}\)) by injecting an animal with human serum. A fluorescent dye is then chemically attached to the anti-human gamma globulin (anti-HGG) antibodies.
The FTA-ABS test (Fluorescent Treponemal Antibody Absorption Test) for syphilis is an example of an indirect fluorescent antibody procedure. This is a confirming test for syphilis since it tests specifically for antibodies in the patient's serum made in response to the syphilis spirochete, Treponema pallidum.
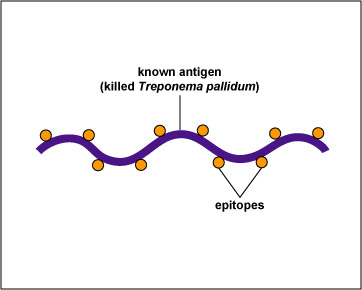
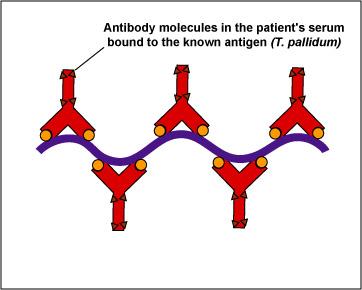
In this test, killed T. pallidum,(the known antigen), is fixed on a slide (see Fig \(\PageIndex{2}\), step 1). The patient's serum is added to the slide. If the patient has syphilis, antibodies against the T. pallidum will react with the antigen on the slide (Fig. \(\PageIndex{2}\), step 2). The slide is then washed to remove any antibodies not bound to the spirochete.
|
Fig. \(\PageIndex{2}\), Step 1 |
Fig. \(\PageIndex{2}\), Step 2 |
|---|---|
|
|
| Treponema pallidum, the known antigen, is fixed to a microscope slide | If there are antibodies against Treponema pallidum in the patient's serum, they will bind to the spirochete. All other antibodies are washed from the slide |
| Copyright; Gary E. Kaiser, Ph.D. The Community College of Baltimore County, Catonsville Campus CC-BY-3.0 | |
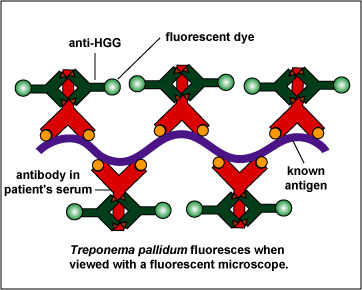
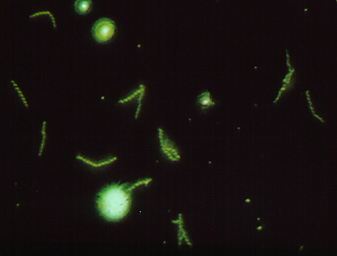
To make this reaction visible, a second animal-derived antibody made against human antibodies and labelled with a fluorescent dye (fluorescent anti-human gamma globulin) is added. These fluorescent anti-HGG antibodies react with the patient's antibodies which have reacted with the T. pallidum on the slide (Fig. \(\PageIndex{2}\), step 3). The slide is washed to remove any unbound fluorescent anti-HGG antibodies and observed with a fluorescent microscope. If the spirochetes glow or fluoresce (see Fig. \(\PageIndex{3}\)), the patient has made antibodies against T. pallidum and has syphilis.
|
Fig. \(\PageIndex{2}\), Step 3FTA Test for Antibodies Against Treponema pallidum |
Fig. \(\PageIndex{3}\): A Positive FTATest for Syphilis Viewed with a Flourescent Microscope |
|
|
| Fluorescent anti-human gamma globulin (anti-HGG) is added to the well. (Anti-HGG is an antibody made by another animal against human IgG antibodies. A fluorescent dye is then attached to the antibody.) The anti-HGG will with any human IgG antibodies bound to the Treponema pallidum on the slide. All unbound anti-HGG is then washed from the slide. When viewed with a flourescent microscope, the spirochetes will fluoresc |
Note the fluorescing spirochetes. |
| Copyright; Gary E. Kaiser, Ph.D. The Community College of Baltimore County, Catonsville Campus CC-BY-3.0 | |
Animation showing the FTA-ABS test for syphilis.
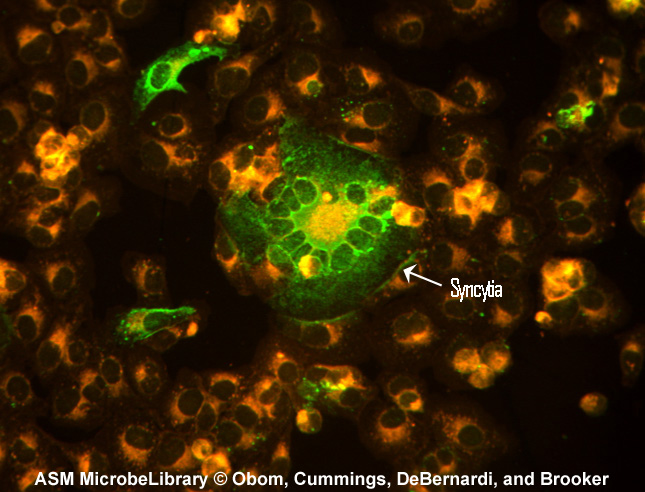
Another example of the indirect fluorescent antibody test is the test for antibodies against the measles virus. Inactivated measles virus-infected cells (the known antigen) are fixed to a microscope slide. The patient's serum is then added. If the person has measles, antibodies of the isotype IgG will be made against the measles virus and will bind to viral epitopes on the know measles virus-infected cells. After washing the slide to remove any unbound IgG, fluorescent antihuman IgG is added. The fluorescent antihuman IgG then binds to the patient's IgG that is bound to the infected cells. When viewed with a fluorescent microscope, the infected cells will fluoresce green as seen in Fig. \(\PageIndex{4}\).
Contributors and Attributions
Dr. Gary Kaiser (COMMUNITY COLLEGE OF BALTIMORE COUNTY, CATONSVILLE CAMPUS)


