16.1: Introduction to Serologic Testing
- Page ID
- 122727
A. INTRODUCTION TO SEROLOGIC TESTING
The adaptive immune responses refer to the ability of the body (self) to recognize specific foreign antigens (non-self) that threaten its biological integrity. There are two major branches of the adaptive immune responses:
1. Humoral immunity: humoral immunity involves the production of antibody molecules in response to an antigen and is mediated by B-lymphocytes.
2. Cell-mediated immunity: Cell-mediated immunity involves the production of cytotoxic T-lymphocytes, activated macrophages, activated NK cells, and cytokines in response to an antigen and is mediated by T-lymphocytes.
To understand the immune responses we must first understand what is meant by the term antigen. Technically, an antigen is defined as a substance that reacts with antibody molecules and antigen receptors on lymphocytes. An immunogen is an antigen that is recognized by the body as nonself and stimulates an adaptive immune response. For simplicity, both antigens and immunogens are usually referred to as antigens.
Chemically, antigens are large molecular weight proteins (including conjugated proteins such as glycoproteins, lipoproteins, and nucleoproteins) and polysaccharides (including lipopolysaccharides). These protein and polysaccharide antigens are found on the surfaces of viruses and cells, including microbial cells (bacteria, fungi, protozoans) and human cells.
As mentioned above, the B-lymphocytes and T-lymphocytes are the cells that carry out adaptive immune responses. The body recognizes an antigen as foreign when that antigen binds to the surfaces of B-lymphocytes and T-lymphocytes by way of antigen-specific receptors having a shape that corresponds to that of the antigen, similar to interlocking pieces of a puzzle. The antigen receptors on the surfaces of B-lymphocytes are antibody molecules called B-cell receptors or sIg; the receptors on the surfaces of T-lymphocytes are called T-cell receptors (TCRs).
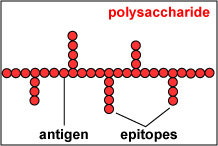
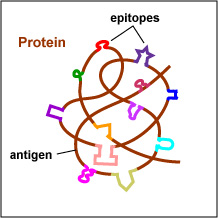
The actual portions or fragments of an antigen that react with receptors on B-lymphocytes and T-lymphocytes, as well as with free antibody molecules, are called epitopes. The size of an epitope is generally thought to be equivalent to 5-15 amino acids or 3-4 sugar residues. Some antigens, such as polysaccharides, usually have many epitopes, but all of the same specificity. This is because polysaccharides may be composed of hundreds of sugars with branching sugar side chains, but usually contain only one or two different sugars. As a result, most "shapes" along the polysaccharide are the same (see Fig. \(\PageIndex{1}\). Other antigens such as proteins usually have many epitopes of different specificities. This is because proteins are usually hundreds of amino acids long and are composed of 20 different amino acids. Certain amino acids are able to interact with other amino acids in the protein chain and this causes the protein to fold over upon itself and assume a complex three-dimensional shape. As a result, there are many different "shapes" on the protein (see Fig. \(\PageIndex{2}\). That is why proteins are more immunogenic than polysaccharides; they are chemically more complex.
A microbe, such as a single bacterium, has many different proteins (and polysaccharides) on its surface that collectively form its various structures, and each different protein may have many different epitopes. Therefore, immune responses are directed against many different parts or epitopes of the same microbe.
|
Fig. \(\PageIndex{1}\): Epitopes of an Antigen (Polysaccharide) |
Fig. \(\PageIndex{2}\: Epitopes of an Antigen (Protein) |
|---|---|
|
|
|
Polysaccharides have many epitopes but of similar specificities |
Proteins have many epitopes of different specificitie |
| Copyright; Gary E. Kaiser, Ph.D. The Community College of Baltimore County, Catonsville Campus CC-BY-3.0 | |
In terms of infectious diseases, the following may act as antigens:
1.Microbial structures (cell walls, capsules, flagella, pili, viral capsids, envelope-associated glycoproteins, etc.); and
2. Microbial toxins
Certain non-infectious materials may also act as antigens if they are recognized as "non-self" by the body. These include:
1. Allergens (dust, pollen, hair, foods, dander, bee venom, drugs, and other agents causing allergic reactions);
2. Foreign tissues and cells (from transplants and transfusions); and
3. The body's own cells that the body fails to recognize as "normal self" (cancer cells, infected cells, cells involved in autoimmune diseases).
Antibodies or immunoglobulins are specific protein configurations produced by B-lymphocytes and plasma cells in response to a specific antigen and capable of reacting with that antigen. Antibodies are produced in the lymphoid tissue and once produced, are found mainly in the plasma portion of the blood (the liquid fraction of the blood before clotting). Serum is the liquid fraction of the blood after clotting.
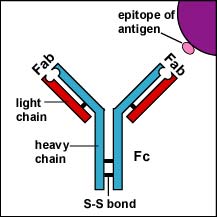
There are 5 classes of human antibodies: IgG, IgM, IgA, IgD, and IgE. The simplest antibodies, such as IgG, IgD, and IgE, are "Y"-shaped macromolecules called monomers composed of four glycoprotein chains. There are two identical heavy chains having a high molecular weight that varies with the class of antibody. In addition, there are two identical light chains of one of two varieties: kappa or gamma. The light chains have a lower molecular weight. The four glycoprotein chains are connected to one another by disulfide (S-S) bonds and noncovalent bonds (see Fig. 3A). Additional S-S bonds fold the individual glycoprotein chains into a number of distinct globular domains. The area where the top of the "Y" joins the bottom is called the hinge. This area is flexible to enable the antibody to bind to pairs of epitopes various distances apart on an antigen.
The two tips of the "Y" monomer are referred to as the Fab portions of the antibody (see Fig. \(\PageIndex{3A}\)). The first 110 amino acids or first domain of both the heavy and light chain of the Fab region of the antibody provide specificity for binding an epitope on an antigen. The Fab portions provide specificity for binding an epitope on an antigen. The bottom part of the "Y" is called the Fc portion and this part is responsible for the biological activity of the antibody (see Fig. \(\PageIndex{3A}\)). Depending on the class of antibody, biological activities of the Fc portion of antibodies include the ability to activate the complement pathway (IgG & IgM), bind to phagocytes (IgG, IgA), or bind to mast cells and basophils (IgE).
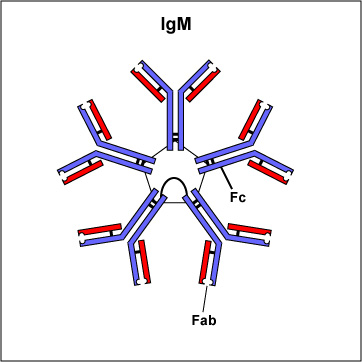
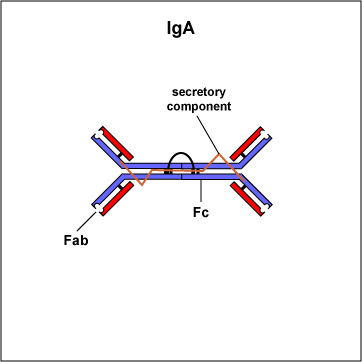
Two classes of antibodies are more complex. IgM is a pentamer (see Fig. \(\PageIndex{3B}\)), consisting of 5 "Y"-like molecules connected at their Fc portions, and secretory IgA is a dimer consisting of 2 "Y"-like molecules (see Fig. \(\PageIndex{3C}\)).
|
Fig. \(\PageIndex{3A}\): IgG |
Fig. \(\PageIndex{3B}\): IgM |
Fig. \(\PageIndex{3C}\): Secretory IgA |
|---|---|---|
|
|
|
|
The Fab portion of the antibody has specificity for binding an epitope of an antigen. The Fc portion directs the biological activity of the antibody |
IgM is a pentamer and, therefore, has 10 Fab sites. | Secretory IgA is a dimer and has 4 Fab sites. A secretory component helps protect it from digestion in body secretions. |
| Copyright; Gary E. Kaiser, Ph.D. The Community College of Baltimore County, Catonsville Campus CC-BY-3.0 | ||
For more information on antigens, antibodies, and antibody production, see the following CourseArc lessons:
Serology refers to using antigen-antibody reactions in the laboratory for diagnostic purposes. Its name comes from the fact that serum, the liquid portion of the blood where antibodies are found is used in testing. Serologic testing may be used in the clinical laboratory in two distinct ways:
a. To identify unknown antigens (such as microorganisms). This is called direct serologic testing. Direct serologic testing uses a preparation known antibodies, called antiserum, to identify an unknown antigen such as a microorganism.
b. To detect antibodies being made against a specific antigen in the patient's serum. This is called indirect serologic testing. Indirect serologic testing is the procedure by which antibodies in a person's serum being made by that individual against an antigen associated with a particular disease are detected using a known antigen.
Contributors and Attributions
Dr. Gary Kaiser (COMMUNITY COLLEGE OF BALTIMORE COUNTY, CATONSVILLE CAMPUS)


