8.5: Indole and Hydrogen Sulfie Production w. Procedures and Results
- Page ID
- 123381
Sometimes we look for the production of products produced by only a few bacteria. As an example, some bacteria use the enzyme tryptophanase to convert the amino acid tryptophan into molecules of indole, pyruvic acid and ammonia. Since only a few bacteria contain tryptophanase, the formation of indole from a tryptophan substrate can be another useful diagnostic tool for the identification of an organism. Indole production is a key test for the identification of Escherichia coli. (See Fig.
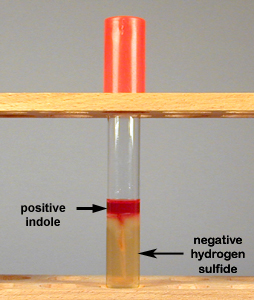
By adding Kovac's reagent to the medium after incubation we can determine if indole was produced. Kovac's reagent will react with the indole and turn red (see Fig. ).
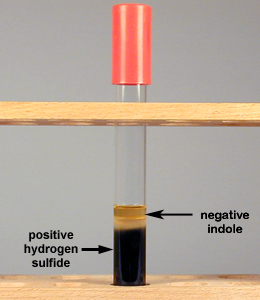
Likewise, some bacteria are capable of breaking down sulfur containing amino acids (cystine, methionine) or reducing inorganic sulfur-containing compounds (such as sulfite, sulfate, or thiosulfate) to produce hydrogen sulfide (H2S). This reduced sulfur may then be incorporated into other cellular amino acids, or perhaps into coenzymes. The ability of an organism to reduce sulfur-containing compounds to hydrogen sulfide can be another test for identifying unknown organisms such as certain Proteus and Salmonella. To test for hydrogen sulfide production, a medium with a sulfur-containing compound and iron salts is inoculated and incubated. If the sulfur is reduced and hydrogen sulfide is produced, it will combine with the iron salt to form a visible black ferric sulfide (FeS) in the tube (see Fig. ).
If neither hydrogen sulfide nor indole are produced in SIM medium, the agar does not turn black and the Kovac's reagent remains yellow. (Fig. ).
|
Fig. : Uninoculated SIM Medium |
Fig. : Production of Indole but No Hydrogen Sulfide in SIM Medium |
Fig. : Production of Hydrogen Sulfide but No Indole in SIM Medium |
Fig. : Negative Hydrogen Sulfide and Negative Indole in SIM Medium |
|---|---|---|---|
 |
 |
||
| SIM medium before inoculation. | If indole is produced from the breakdown of the amino acid tryptophan, the Kovac's reagent, when added, will turn red. If hydrogen sulfide is not produced, the agar does not turn black. | SIM medium turns black if hydrogen sulfide is produced from the reduction of sulfur. If Kovac's reagent remains yellow, indole is not produced. | ndole negative, because the Kovac's reagent did not turn red, and hydrogen sulfide negative, because the agar did not turn black |
| Copyright; Gary E. Kaiser, Ph.D. The Community College of Baltimore County, Catonsville Campus CC-BY-3.0 | |||
Three tubes of SIM (Sulfide, Indole, Motility) medium . This medium contains a sulfur source, an iron salt, the amino acid tryptophan, and is semi-solid in agar content (0.3%). It can be used to detect hydrogen sulfide production, indole production, and motility.
Trypticase Soy agar cultures of Proteus mirabilis, Escherichia coli, and Enterobacter cloacae.
PROCEDURE (to be done in pairs)
1. Stab one SIM medium tube with Proteus mirabilis.
2. Stab a second SIM medium tube with Escherichia coli.
3. Stab a third SIM medium tube with Enterobacter cloacae.
4 . Incubate the tubes in your test tube rack on your shelf of the 37°C incubator corresponding to your lab section until the next lab period
5. Next lab period add Kovac's reagent to each tube to detect indole production.
Results
Carefully add about 1/4 inch of Kovac's reagent to each of the 3 SIM agar tubes and observe.
1. Production of hydrogen sulfide (H2S)
- If the bacterium produces the enzyme to reduce sulfur to hydrogen sulfide (H2S), the agar will turn black indicating that the organism has produced hydrogen sulfide (see Fig. ).
- If the bacterium lacks the enzyme, the agar does not turn black, indicating that hydrogen sulfide was not produced.
2. Production of indole
- If the bacterium produces the enzyme to break down tryptophan into molecules of indole, pyruvic acid, and ammonia, the Kovac's reagent will turn red, indicating the organism is indole-positive (see Fig. ).
- If the Kovac's reagent remains yellow, no indole was produced and the organism is indole-negative (see Fig. ).
Record your results below (+ = positive; - = negative).
| Organism | Indole | Hydrogen Sulfide |
|---|---|---|
| Escherichia coli | ||
| Enterobacter cloacae | ||
| Proteus mirabilis |
Contributors and Attributions
Dr. Gary Kaiser (COMMUNITY COLLEGE OF BALTIMORE COUNTY, CATONSVILLE CAMPUS)

