37: IMViC tests
- Page ID
- 3669
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Understand the importance of these 4 IMViC tests, plus the addition SIM tests
- Determine the various reactions for these media: MRVP broth, SIM, Simmon citrate, and SIM.
IMViC: Indole, Methyl red, Voges-Proskauer, Citrate + and H2S
These 4 IMViC tests (actually 6 tests if you include motility and H2S) constitute, perhaps, the most critical tests used for identification of bacteria after the gram stain. The test results from these 6 tests should carry more weight than almost any other tests, certainly higher priority than sugar results since they are more stable reactions.
The SIM agar deep is a multi-test medium comprising 3 tests: sulfide (H2S gas), indole production, and motility. You have already tested for motility via the hanging drop slide and TTC, and here is an additional way to determine it (although not as good as TTC). The amino acid tryptophan can be converted by the enzyme tryptophanase into an end product called indole. This chemical is identified when it reacts with Kovac's reagent. Since the TTC agar deep is a better way to run motility, we use SIM for indole and sulfide production. Sodium thiosulfate is in this medium and can be utilized by bacteria, with the production of hydrogen sulfide. H2S is colorless. Ferric ammonium sulfite is in the medium to reaction with H2S, producing a black ferrous sulfide.
The MRVP tests are run together in the same broth and then split into 2 tubes when ready to be tested for the end products. The methyl red test determines the use of glucose, with the subsequent production of acid, tested for by the pH indicator methyl red. The Voges-Proskauer test also determines glucose use, but for a different end product---not acid but a neutral product called acetoin (or acetylmethylcarbinol). The VP is really important for identification of many bacteria, but it is a picky test, and MUST be done exactly right.
The citrate test identifies the use of citrate as a sole carbon source, since there are no other nutrients in this medium. The basic end products (carbonates, bicarbonates, and ammonium hydroxide) will cause the brom thymol blue indicator in the medium to turn from forest green to royal blue.
MATERIALS NEEDED
- 1 SIM deep
- 1 MRVP broth
- 1 Simmon citrate agar slants
- TTC motility agar deep
AFTER INCUBATION: reagents
- Kovac's reagent
- Barritt's reagents A (alpha-naphthol) and B (KOH)
- methyl red reagent
THE PROCEDURES
Indole test, H2S, and motility
- Inoculate bacterium into a tube of SIM media with a NEEDLE, all the way to the bottom. YOU MAY PREFER TO USE THE TTC MOTILITY AGAR FOR MOTILITY because it is easier to interpret.
- Incubate at 37º C.
Methyl red and Voges-Proskauer tests
- Inoculate bacterium into the MRVP broth.
- Incubate at 37º C.
Citrate test
- Streak up the slant with the inoculum.
- Incubate at 37º C.
INTERPRETATION: AFTER INCUBATION
SIM: Indole test, H2S, and motility
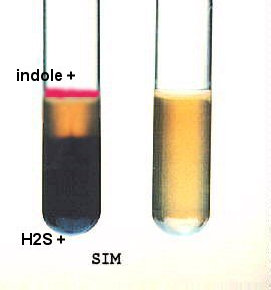 Look for H2S FIRST, a black precipitate within the agar deep.
Look for H2S FIRST, a black precipitate within the agar deep.- For SIM: Hold the tube up to the light and look at the stab line to determine motility. If NONMOTILE, you will see the intact straight stab line. If MOTILE, the original stab line will diffuse out into the medium as the bacteria spread throughout. In fact, you may not even be able to see a stab line at all: it may be turbid throughout the medium. FOR THAT REASON, you might want to compare the inoculated SIM with an uninoculated one.
- For TTC: Look for the spread of the RED color away from the stab inoculation/ growth line. Motile organisms will SPREAD away from the stab line, while nonmotile do not. Examples of motile and nonmotile in this medium can be seen in the MOTILITY exercise.
- After reading both motility and H2S, add 6-8 drops of Kovac's reagent to detect the presence of indole produced from tryptophan breakdown. Within just a few seconds you can see the hot pink color of indole presence.
Methyl Red and Voges-Proskauer: AFTER INCUBATION
- Pour 1/3 of the suspension into a clean nonsterile tube: run the MR test in the tube with 2/3, and the VP test in the open tube with 1/3.
- For methyl red: Add 6-8 drops of methyl red reagent to the tube containing 2/3 of the broth, shake well. This will be an immediate reaction, if positive.
- For Voges-Proskauer: To the tube containing only 1/3 of the solution, add 12 drops of Barritt's A, shake, and 4 drops of Barritt's B, mixing gently for 1 minute to aerate the solution (the reaction requires O2,so no cap on it). Let sit, undisturbed, for at least 30 minutes, maybe more.
Methyl red
Within just a few seconds after adding methyl red reagent, you can see the red-pink color of acid presence from glucose use.

Voges-Proskauer


The reagents MUST be added in the correct order, in the correct amounts, and the tube must sit undisturbed,and open to the air (no cap) for at least 30 minutes (45 minutes is even better) as the light pink color intensifies at the top of the tube (the reagents react with acetoin). DO NOT shake the tube AFTER setting it down for the waiting period.
Note
Some bacteria will have different VP reactions at 30º C vs. 37º C (noted in the identification books). You might want to run this test at different temperatures.
Citrate test
The alkaline by-products of citrate use will cause the pH indicator to turn royal blue.

Note
Be sure that you see both – and + test results of all of these tests by visiting other tables in the lab.
QUESTIONS
- What are the reagents used in the indole test, methyl red test, and Voges-Proskauer test?
- The purpose of the citrate in the Simmon citrate medium is to determine if the organism can use citrate as the sole ___________ source.
- Why determine motility and H2S before adding Kovac's reagent?
- What is so picky about the Voges-Proskaeur test?
- The end product identified in the VP test is a neutral compound called ______.

