Enzymes
- Page ID
- 2853
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)This laboratory exercise will explore the effect of temperature, pH, and enzyme concentration on the rate of a reaction.
Part A- Temperature
Higher temperature generally causes more collisions among the molecules and therefore increases the rate of a reaction. More collisions increase the likelihood that substrate will collide with the active site of the enzyme, thus increasing the rate of an enzyme-catalyzed reaction. Above a certain temperature, activity begins to decline because the enzyme begins to denature. The rate of chemical reactions therefore increases with temperature but then decreases as enzymes denature. This part of the exercise will explore the rate of enzyme activity at two different temperatures. It will also explore the rate of enzyme activity using an enzyme that has been denatured.
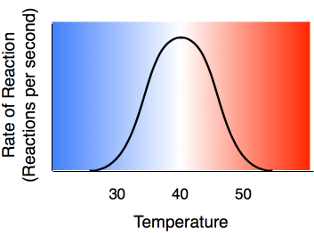
Rennin is an enzyme found in the stomach of mammals where it functions to solidify milk. You will observe the activity of this enzyme by mixing it with milk in a test tube. The presence of a reaction is indicated by milk becoming solid.
A1. Predict what will happen in each of the tubes described in the two experiments described below and write your two hypotheses in your notebook. The word "milk" should appear in each of your hypotheses. You should have two hypotheses- one that addresses the the effect of temperature on rate of reaction (tubes 1 and 3) and one that addresses the effect of denaturing the enzyme on reaction time (tubes 3 and 5). Your hypotheses should be specific for this experiment; it should state the expected outcome of this experiment.
Be sure to write your hypotheses as statements, not as questions. At this point, you do not know what will happen; your hypotheses may be correct or incorrect. An incorrect hypothesis is perfectly acceptable. You will test the hypotheses by performing the experiment below.
Effect of Temperature
A1. Write a hypothesis for this experiment in your lab notebook. The word "milk" should be used in your hypothesis. For example: "The milk in tube #1 will...."
A2. Obtain five test tubes and put a mark 2 cm from the bottom of each using a wax pencil. Four of these test tubes will be used in this experiment (Effect of Temperature) and two of them will be used in the next experiment (Effect of Denaturing the Enzyme, below).
A3. Mark the tubes with your initials or some other means to identify them. The tubes should also be numbered 1 through 5.
A4. Fill all of the tubes to the 2 cm mark with milk.
A5. Add 3 drops of rennin to the milk in tube #1 and keep it at room temperature for 15 minutes. Tube #2 without rennin should also be kept at room temperature to serve as a control.
A6. Add 3 drops of rennin to the milk in tube #3 and place it in a 37 degree incubator for 15 minutes. Tube #4 without rennin should also be placed in the incubator for 15 minutes to serve as a control.
Effect of Denaturing the Enzyme
A7. Place a mark at 1 cm on a test tube and add rennin up to the mark. Place the tube in a boiling water bath for 2 minutes. After boiling, pour the rennin in a small beaker.
A8. Add 3 drops of boiled rennin to the milk in tube #5 and place it in the 37 degree incubator for 15 minutes along with the tubes prepared in step #A6 above. Tube #4 prepared earlier will serve as a control in this experiment as well as a control in the previous experiment (step A6 above).
The two experiments that you conducted in Part A are summarized below. Tubes 1 through 4 are used to investigate the effect of temperature on enzyme activity. Tubes 4 and 5 are used to investigate the effect of denaturing an enzyme with heat and then using the enzyme at its normal temperature (body temperature).
|
Effect of Temperature
Room Temperature:
37 Degrees: |
Tube 1, Tube 2 (control) Tube 3, Tube 4 (control) |
|
Effect of Denaturing the Enzyme:
|
Tube 5, Tube 6 (control) |
A9. Observe the milk in each of the five tubes after the 15 minute period and record your observations in your notebook.
A10. Do your results support either of your hypotheses? Explain.
Part B- pH
Each enzyme has an optimal pH.
A change in pH can alter the ionization of the R groups of the amino acids. When the charges on the amino acids change, hydrogen bonding within the protein molecule change and the molecule changes shape. The new shape may not be effective.
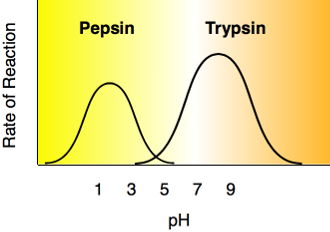
The diagram below shows that pepsin functions best in an acid environment. This makes sense because pepsin is an enzyme that is normally found in the stomach where the pH is low due to the presence of hydrochloric acid. Trypsin is found in the duodenum, and therefore, its optimum pH is in the neutral range to match the pH of the duodenum.
Most cells form hydrogen peroxide (H2O2) as a waste product of aerobic respiration. Hydrogen peroxide is toxic and must be converted to water and oxygen by the enzyme catalase.
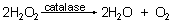
Hydrogen peroxide is also commonly used as a household disinfectant. It bubbles when it is applied to cuts and scrapes because catalase is present in the fluids of the broken cells. As the equation above shows, the bubbles are oxygen gas (O2).
In the experiment below, bubbling will be used as an indication that a reaction is occurring. A faster reaction will have more bubbling.
B1. Predict how pH will affect the rate of reaction in the three different situations described below and write your hypothesis or hypotheses in your notebook. At this point, you do not know if your hypothesis is true or false. It is acceptable to create a hypothesis which will be shown to be false.
B2. Mark three test tubes with a wax pencil 3 cm from the bottom and 6 cm from the bottom.
B3. Add 3 cm of hydrogen peroxide to each tube.
B4. Fill one of the tubes to the 6 cm mark with 5 m HCl.
B5. Fill another tube to the 6 cm mark with 5 m NaOH.
B6. Fill the third tube to the 6 cm mark with distilled water.
B7. Cut three strips of potato that are approximately 3 cm long. The strips should ne cut thin enough to easily fit into a test tube.
B8. Place one of the strips of potato into each of the three tubes that you prepared in steps 2-6 above.
B9. Write your observation of the amount of bubbling in your notebook. We are only interested in the amount of bubbling. We are not interested in any change in color or whether the potato floats or sinks.
B10. Use pH paper to measure the pH of each tube and record your measurements in your notebook.
B11. At what pH did catalase function best in this experiment?
B12. Do your results support your hypothesis? Explain.
B13. In part A of this lab, you worked with rennin, an enzyme found in the stomach of mammals. The pH of the stomach is normally about 2.0. At what pH do you predict rennin works best?
B14. What can you say about pH and enzyme functioning? Is there a single pH that enzymes function best at or does it depend on the enzyme?
Part C- Substrate Concentration
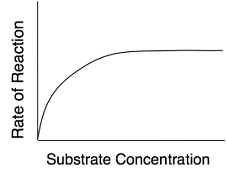
At lower concentrations, the active sites on most of the enzyme molecules are not filled because there is not much substrate. Higher concentrations cause more collisions between the molecules. With more molecules and more collisions, enzymes are more likely to encounter molecules of reactant.
The maximum velocity of a reaction is reached when the active sites are almost continuously filled. Increased substrate concentration after this point will not increase the rate. Reaction rate therefore increases as substrate concentration is increased but it levels off.
Enzyme Concentration
If there is insufficient enzyme present, the reaction will not proceed as fast as it otherwise would because all of the active sites are occupied with the reaction. Additional active sites could speed up the reaction. As the amount of enzyme is increased, the rate of reaction increases.
In this investigation, we will examine what happens to the rate of a reaction when the amount of enzyme is reduced. We will use urease, an enzyme that converts urea to ammonia. The ammonia causes the pH of the water to increase (it becomes more basic). You will be able to tell when a reaction occurs because the urea solution also contains a pH indicator that is becomes yellow in acid but turns red when the solution becomes basic.
The object of this experiment is to measure the amount of time it takes for the solution to turn red if less enzyme is used.
C1. Create a hypothesis regarding the the amount of urease and the rate of reaction of Urea.
C2. Obtain four test tubes and rinse them thoroughly using distilled water.
C3. Add 2 cm of urea to each.
C4. Label three of these tubes 1 through 3; the remaining tube will not be used; it will serve as a control.
C5. Have your lab partner start timing as you add 20 drops of urease to tube #1. Swirl the tube, then place it in a test tube rack. Record the amount of time that it takes for the solution to change to a red color.
C6. Add 10 drops of urease to tube #2, swirl the tube, then place it in a rack. Record the amount of time that it takes for the solution to change to a red color.
C7. Add 5 drop of urease to tube #3, swirl the tube, swirl the tube, then place it in a rack. Record the amount of time that it takes for the solution to change to a red color.
C8. Record your results in your notebook.
C9. Did using less enzyme produce a reaction?
C10. What was the effect of using less enzyme in your experiment? If your experiment did not work as expected, what should have happened?
C11. In general, what happens to the rate of reaction as the amount of enzyme is decreased?
C12. Do your results support your hypothesis? Explain.

