17: Gram Stain
- Page ID
- 3624
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Objectives
- Become proficient at performing the gram stain consistently and accurately.
- Differentiate among various shapes, sizes, arrangements, and gram reactions of bacteria.
The gram stain, originally developed in 1884 by Christian Gram, is probably the most important procedure in all of microbiology. It has to be one of the most repeated procedures done in any lab. Gram was actually using dyes on human cells, and found that bacteria preferentially bind some dyes. The Gram stain is a differential stain, as opposed to the simple stain which uses 1 dye. As a result of the use of 2 dyes, making this procedure a differential stain, bacteria will either become purple/blue or pink during the procedure.
Before staining, the specimen must be mounted and fixed on the slides, as previously done in the simple staining technique. Because of the 2 dyes used in the procedure--crystal violet and safrinin---as well as the decolorizer acetone-alcohol, bacteria will fall into 2 groups based on their gram reactivity. Gram positive bacteria retain the crystal violet even through the decolorizor step: gram negative bacteria do not retain the crystal violet, are decolorized, and then pick up the safrinin dye. Both gram + and – bind to the crystal violet: the key step to their differentiation is the decolorization.
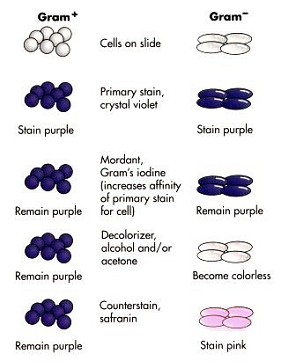 Take a look at the accompanying diagram of the stain procedure and its effects on the bacterial color. During the crystal violet-iodine step, the bound molecules within the peptidoglycan of the gram + cell wall and within the membrane are held tightly. The acetone-alcohol actually causes the peptidoglycan molecules (arranged in a latticework) to shrink, thereby holding the crystal violetiodine even tighter. In the gram – cell, the outer lipopolysaccharide layer of the wall is dissolved by the decolorizer agents, and because the peptidoglycan layer is so thin in that group of bacteria, the crystal violet is leached out of the wall.
Take a look at the accompanying diagram of the stain procedure and its effects on the bacterial color. During the crystal violet-iodine step, the bound molecules within the peptidoglycan of the gram + cell wall and within the membrane are held tightly. The acetone-alcohol actually causes the peptidoglycan molecules (arranged in a latticework) to shrink, thereby holding the crystal violetiodine even tighter. In the gram – cell, the outer lipopolysaccharide layer of the wall is dissolved by the decolorizer agents, and because the peptidoglycan layer is so thin in that group of bacteria, the crystal violet is leached out of the wall.
Although there is a standard routine and set reagents used in this stain, each person has to find a particular method that works best for them. The many variables that can affect this stain are age of the culture, amount of decolorizer used, the time of decolorization, the type of organism (acid-fast bacteria and spores do not stain well), thickness of the smear, and the general care of the stainer.
The most common reasons for false gram reactions?
- Some bacterial species tend towards gram variable, and will show both colors although most often gram +.
- Over decolorizing the smear, too long a time.
- Using old cultures (preferably, the cultures should be 18-48 hours old).
Note
- Make sure that your smear is not too thick: otherwise, either the dyes cannot penetrate or the cells will not be decolorized adequately.
- Be sure that you properly time the decolorization step.
- Flood the smear with decolorizing agent evenly over the smear.
MATERIALS NEEDED
- Gram negative control: E. coli culture
- Gram positive control: Bacillus subtilus culture
- prepared, bought slides of various gram stained bacteria
- dyes
- dye stain tub
- wire overlay for tub
- clean microscope slides
- immersion oil
- blotting paper
THE PROCEDURE: done individually
- We have cultures of E. coli and Bacillus for you to gram stain. This will give you gram + and gram - controls to check your procedure against. You can use 2 slides, 1 for each bacterium, or you can divide one slide in half and smear each bacterium on the divided slide. There are also prepared, gram stained slides of bacteria of different shapes and sizes of bacteria to look at.
- Make the bacterial smear from broth, slant, or plate.
- If taken from a broth, use 1-2 loopfuls of the broth solution.
- If taken from a solid agar medium (plate or slant), suspend the inoculum in a drop of water on the slide and mix it well.
- Spread the suspension on the slide so that it covers an area at least the size of a nickel, preferably a quarter.
- You might think about marking the smear area with a surrounding wax pencil mark so that you can find the smear under the microscope easily.
- Place a piece of tape on the side of the smear so you know which way is UP.
- Let the slide air-dry totally before proceeding on with the procedure.
- Heat-fix the slide by holding the slide at one end with your fingers and quickly moving it back and forth a few times over the flame.
- Perform the Stain procedure as stated below.
The STAIN procedure
- Place the slide on the wire mesh overlay on the dye tub.
- Flood the smear with drops of crystal violet, leaving in for 1 minute. Wash WELL with water.
- Flood the smear with drops of Gram's iodine, leaving in for 1 minute. Wash WELL with water.
- Flood acetone-alcohol quickly on the slide, and wash off within 5-10 seconds (from beginning of decolorizer added). Wash WELL with water.
- Flood the smear with drops of safrinin, leaving in for 1 minute. Wash WELL with water.
- Blot dry with bibulous paper before placing on microscope stage to view.
- Focus on smear using low power lens, ending up on 100X oil immersion. Be sure that you have a drop of oil on the slide before rotating your 100X objective lens into place.
- Interpret the results using the protocol below.
Interpretation
- Gram positive bacteria will be blue/ purple/ violet
- Gram negative bacteria will be light pink
- Identify the various shapes and arrangements of bacteria.
- CLEAN YOUR SLIDES using the slide cleanser at the sinks.
- Look at the prepared slides of various bacteria. You will see different shapes and arrangements. Return prepared slides to the trays on the bench.
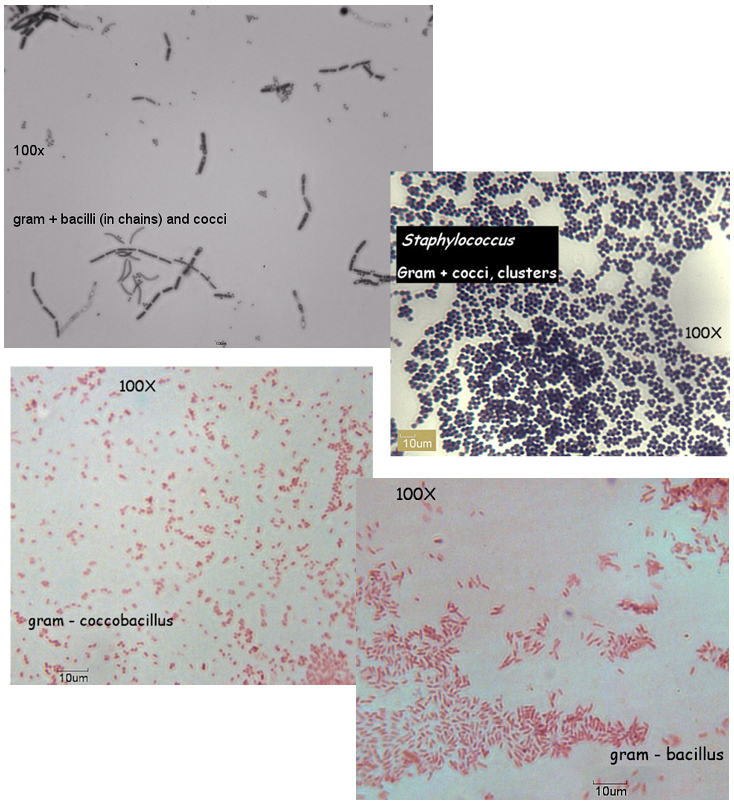
Various bacteria slides
QUESTIONS
- Critiquing your gram stain technique:
- Are the cells well distributed on the slide?
- Are the cells stained uniformly and is the gram reaction correct?
- Is the arrangement of the bacterium consistent across the various fields of vision?
- What are the gram reaction, shape, and arrangement of Bacillus?
- What are the gram reaction, shape, and arrangement of E. coli?
- What color is E. coli when gram stained? Name the dye that gives it this color.
- To what cell structure do the 2 dyes bind?
- List at least 3 differences between gram positive and gram negative bacteria.
- Would it be useful to perform a gram stain on a mixed culture? Why?
- If a gram stain gives you some valuable information on features of the bacterium, what would be the benefit of ever performing a simple stain?
Contributors and Attributions
Jackie Reynolds, Professor of Biology (Richland College)

