7.7: Biofuels (Biomass Energy)
- Page ID
- 69429
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Biofuels (biomass energy) contain energy produced from organisms, such as animal waste, plants, or algae. It is another indirect form of solar energy. Biofuels have many uses. They be burned directly or first converted to ethanol (often with the help of bacteria and fungi) to generate electricity. The heat from combustion produces steam and turns a turbine to power a generator. Biodiesel offers an alternative to petrochemicals for fueling vehicles. Biofuels have even been used to power small planes (figure \(\PageIndex{a}\)). Furthermore, burning wood or straw provides heating.

Figure \(\PageIndex{a}\): Test flight of the "Green Hornet", a Navy fighter jet partially powered by biofuels. Image by U.S. Navy (public domain).
Unlike fossil fuels, biofuels are carbon neutral (figure \(\PageIndex{b}\)). Fossil fuels store carbon that was captured by organisms millions of years ago. When we burn them, carbon dioxide is released much more rapidly than it was removed. Biofuels removed carbon dioxide from the atmosphere more recently, and they form over shorter time spans. When biofuels are burned, this carbon dioxide that was recently removed is released back into the atmosphere.
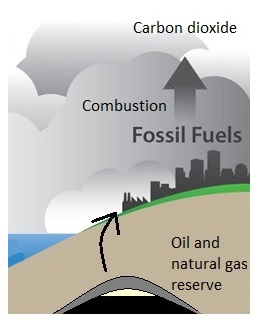
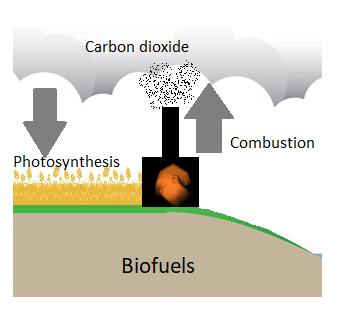
Figure \(\PageIndex{b}\): Fossil fuels, such as oil and natural gas, contain carbon that has been stored underground for millions of years. Combustion (burning) of these fossil fuels releases carbon dioxide into the atmosphere, contributing to climate change. When biofuels are grown, they capture carbon dioxide through photosynthesis. Burning them or their products for heat, transportation, or electricity generation release this carbon dioxide, which was only recently captured. For this reason, biofuels are considered carbon neutral. Images created by Melissa Ha from NETL/DOE (public domain) and publicdomainvectors.org (public domain).
Another advantage of biofuels is that they can be produced locally and can be cultivated in many different locations. On the other hand, they occupy space that could otherwise be used for food production. To further complicate matters, the characteristics that make a plant species ideal for biofuel (such as being resistant to pests and fast-growing) are also characteristics that help invasive species thrive. Care must taken to contain these species if grown outside of their native ranges.
Combustion of solid municipal waste (see below) or animal wastes as biofuels, reduces waste and generates electricity simultaneously. Unlike most forms of renewable energy, however, combustion of biofuels pollutes the air. (The carbon dioxide released is not an issue since biofuels are carbon neutral, but other air pollutants are also released.) In fact, indoor air pollution from fires used for cooking inside the home is a major cause of death in developing countries.
Each type of biomass must be evaluated for its environmental and social impact in order to determine if it is really advancing sustainability and reducing environmental impacts. For example, cutting down large swaths of forests just for energy production is not a sustainable option because our energy demands are so great that we would quickly deforest the world, destroying critical habitat. For biomass to be a sustainable option, it usually needs to come from waste material, such as lumber mill sawdust, paper mill sludge, yard waste, or oat hulls from an oatmeal processing plant, livestock manure, or trash. These materials would otherwise just accumulate or decompose. Several examples of biofuel use are discussed below in more detail, including the specific advantages and disadvantages of type of use.
Burning Wood
Using wood, and charcoal made from wood, for heating and cooking can replace fossil fuels and may result in lower carbon dioxide emissions. If wood is harvested from forests or woodlots that have to be thinned or from urban trees that fall down or needed be cut down anyway, then using it for biomass does not impact those ecosystems. However, wood smoke contains harmful pollutants like carbon monoxide and particulate matter (see Air Pollution).
For home heating, it is most efficient and least polluting when using a modern wood stove or fireplace insert that are designed to release small amounts of particulates. However, in places where wood and charcoal are major cooking and heating fuels such as in developing countries, the wood may be harvested faster than trees can grow resulting in deforestation (figure \(\PageIndex{c}\)). The largest share of biofuel use comes from traditional biomass, mostly fuel wood gathered for household cooking and heating, often without regard for sustainable replacement.

Figure \(\PageIndex{c}\): Charcoal production in Uganda. (A) Trees cut down for charcoal. (B) Pieces of firewood. (C) Wood logs read for charcoal burning. (D) Charcoal packed and ready for market. Image and caption (modified) from Bamwesigye et al. Sustainability 2020, 12(20), 8337. (CC-BY)
Biomass can be used in small power plants. For instance, Colgate College has had a wood-burning boiler since the mid-1980s (figure \(\PageIndex{d}\)). In one year it processed approximately 20,000 tons of locally and sustainably harvested wood chips, the equivalent of 1.17 million gallons (4.43 million liters) of fuel oil, avoiding 13,757 tons of emissions and saving the university over $1.8 million in heating costs. The University’s steam-generating wood-burning facility now satisfies more than 75% of the campus’s heat and domestic hot water needs.

Figure \(\PageIndex{d}\): Wood chips are one example of a biofuel that can be burned to generate electricity. Source: Ulrichulrich
Municipal Solid Waste
Municipal solid waste (MSW) is commonly known as garbage and can create electricity by burning it directly or by burning the methane produced as it decays. Waste-to-energy processes are gaining renewed interest as they can solve two problems at once: disposal of waste and production of energy from a renewable resource. Many of the environmental impacts are similar to those of a coal plant: air pollution, ash generation, etc. Because the fuel source is less standardized than coal and hazardous materials may be present in MSW, incinerators and waste-to-energy power plants need to clean the gases of harmful materials. The Environmental Protection Agency in the U.S. regulates these plants very strictly and requires anti-pollution devices to be installed. Also, while incinerating at high temperature, many of the toxic chemicals may break down into less harmful compounds. The ash from these plants may contain high concentrations of various metals that were present in the original waste. If ash is clean enough it can be “recycled” as an MSW landfill cover or to build roads, cement blocks, and artificial reefs (similar to coral reefs, but built by humans).
Landfill Gas (Biogas)
Landfill gas (biogas) is a sort of human-made “biogenic” gas as discussed above (figure \(\PageIndex{e}\)). Methane is formed as a result of biological processes in sewage treatment plants, waste landfills, anaerobic composting, and livestock manure management systems. This gas is captured and burned to produce heat or electricity. The electricity may replace electricity produced by burning fossil fuels, reducing carbon dioxide emissions. The only environmental impacts are from the construction of the plant itself, similar to that of a natural gas plant.
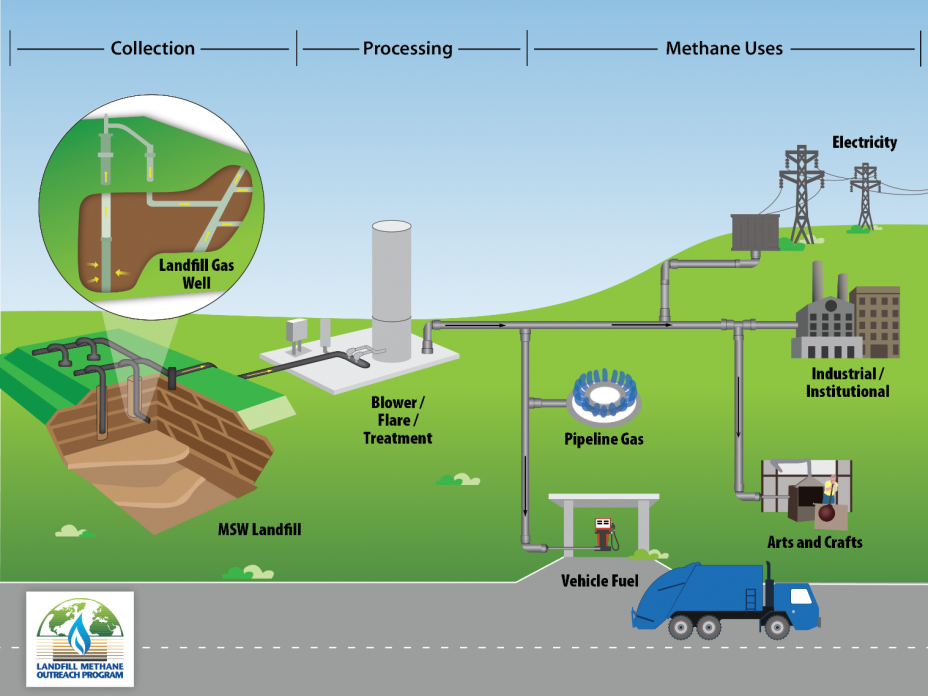
Figure \(\PageIndex{e}\): The collection and processing of landfill gas to produce methane for multiple uses. First, landfill gas is collected from the landfill gas well. A system of vertical and horizontal piping buried in an municipal solid waste landfill. The landfill gas is then processed and treated for use (blower/flare/treatment). Potential end uses include industrial/institutional uses, arts and crafts, pipeline gas, and vehicle fuel. Image and caption (modified) from Landfill Methane Outreach Program/EPA (public domain).
Bioethanol and Biodiesel
Bioethanol and biodiesel are liquid biofuels manufactured from plants, typically crops. Bioethanol can be easily fermented from sugar cane juice, as is done in Brazil. Additionally, it can be fermented from broken down corn starch, as is mainly done in the United States.
The economic and social effects of growing plants for fuels need to be considered, since the land, fertilizers, and energy used to grow biofuel crops could be used to grow food crops instead. The competition of land for fuel versus food can increase the price of food, which has a negative effect on society. It could also decrease the food supply increasing malnutrition and starvation globally. Also, in some parts of the world, large areas of natural vegetation and forests have been cut down to grow sugar cane for bioethanol and soybeans and palm-oil trees to make biodiesel. This is not sustainable land use. Derived biofuels from parts of plants not used for food, such as stalks, reduces their environmental impact. Biodiesel can be made from used vegetable oil and has been produced on a very local basis. Compared to diesel, a petrochemical derived from crude oil, biodiesel combustion produces less sulfur oxides, particulate matter, carbon monoxide, and unburned and other hydrocarbons, but it produces more nitrogen oxide (see Air Pollution).
Liquid biofuels typically replace petroleum and are used to power vehicles (figure \(\PageIndex{f}\)). Although ethanol-gasoline mixtures burn cleaner than pure gasoline, they also are more volatile and thus have higher “evaporative emissions” from fuel tanks and dispensing equipment. These emissions contribute to the formation of harmful, ground level ozone and smog (see Air Pollution). Gasoline requires extra processing to reduce evaporative emissions before it is blended with ethanol.

Figure \(\PageIndex{f}\): A bus running on soybean biodiesel. Image and caption (modified) from U.S Department of Energy (public domain).
Attribution
Modified by Kyle Whittinghill and Melissa Ha from the following sources:
- Renewable Energy and Challenges and Impacts of Energy Use from Environmental Biology by Matthew R. Fisher (licensed under CC-BY)
- Essentials of Environmental Science by Kamala Doršner is licensed under CC BY 4.0.


