4.4: Nucleic Acids
- Page ID
- 8394
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Nucleic acids
There are two types of nucleic acids in biology: DNA and RNA. DNA carries the heritable genetic information of the cell and is composed of two antiparallel strands of nucleotides arranged in a helical structure. Each nucleotide subunit is composed of a pentose sugar (deoxyribose), a nitrogenous base, and a phosphate group. The two strands associate via hydrogen bonds between chemically complementary nitrogenous bases. Interactions known as "base stacking" interactions also help stabilize the double helix. By contrast to DNA, RNA can be either be single stranded, or double stranded. It too is composed of a pentose sugar (ribose), a nitrogenous base, and a phosphate group. RNA is a molecule of may tricks. It is involved in protein synthesis as a messenger, regulator, and catalyst of the process. RNA is also involved in various other cellular regulatory processes and helps to catalyze some key reactions (more on this later). With respect to RNA, in this course we are primarily interested in (a) knowing the basic molecular structure of RNA and what distinguishes it from DNA, (b) understanding the basic chemistry of RNA synthesis that occurs during a process called transcription, (c) appreciating the various roles that RNA can have in the cell, and (d) learning the major types of RNA that you will encounter most frequently (i.e. mRNA, rRNA, tRNA, miRNA etc.) and associating them with the processes they are involved with. In this module we focus primarily on the chemical structures of DNA and RNA and how they can be distinguished from one another.
Nucleotide structure
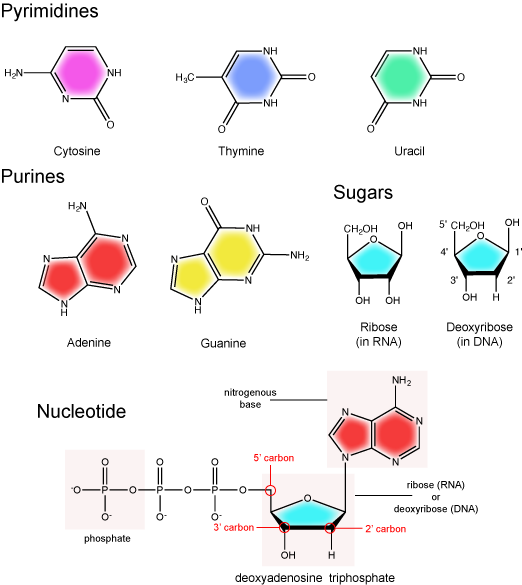
The two main types of nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). DNA and RNA are made up of monomers known as nucleotides. Individual nucleotides condense with one another to form a nucleic acid polymer. Each nucleotide is made up of three components: a nitrogenous base (for which there are five different types), a pentose sugar, and a phosphate group. These are depicted below. The main difference between these two types of nucleic acids is the presence or absence of a hydroxyl group at the C2 position, also called the 2' position (read "two prime"), of the pentose (see Figure 1 legend and section on the pentose sugar for more on carbon numbering). RNA has a hydroxyl functional group at that 2' position of the pentose sugar; the sugar is called ribose, hence the name ribonucleic acid. By contrast, DNA lacks the hydroxyl group at that position, hence the name, "deoxy" ribonucleic acid. DNA has a hydrogen atom at the 2' position.
The nitrogenous base
The nitrogenous bases of nucleotides are organic molecules and are so named because they contain carbon and nitrogen. They are bases because they contain an amino group that has the potential of binding an extra hydrogen, and thus acting as a base by decreasing the hydrogen ion concentration in the local environment. Each nucleotide in DNA contains one of four possible nitrogenous bases: adenine (A), guanine (G), cytosine (C), and thymine (T). By contrast, RNA contains adenine (A), guanine (G) cytosine (C), and uracil (U) instead of thymine (T).
Adenine and guanine are classified as purines. The primary distinguishing structural feature of a purine is double carbon-nitrogen ring. Cytosine, thymine, and uracil are classified as pyrimidines. These are structurally distinguished by a single carbon-nitrogen ring. You will be expected to recognize that each of these ring structures is decorated by functional groups that may be involved in a variety of chemistries and interactions.
Note: practice
Take a moment to review the nitrogenous bases in Figure 1. Identify functional groups as described in class. For each functional group identified, describe what type of chemistry you expect it to be involved in. Try to identify whether the functional group can act as either a hydrogen bond donor, acceptor, or both?
The pentose sugar
The pentose sugar contains five carbon atoms. Each carbon atom of the sugar molecule are numbered as 1′, 2′, 3′, 4′, and 5′ (1′ is read as “one prime”). The two main functional groups that are attached to the sugar are often named in reference to the carbon to whch they are bound. For example, the phosphate residue is attached to the 5′ carbon of the sugar and the hydroxyl group is attached to the 3′ carbon of the sugar. We will often use the carbon number to refer to functional groups on nucleotides so be very familiar with the structure of the pentose sugar.
The pentose sugar in DNA is called deoxyribose, and in RNA, the sugar is ribose. The difference between the sugars is the presence of the hydroxyl group on the 2' carbon of the ribose and its absence on the 2' carbon of the deoxyribose. You can, therefore, determine if you are looking at a DNA or RNA nucleotide by the presence or absence of the hydroxyl group on the 2' carbon atom—you will likely be asked to do so on numerous occasions, including exams.
The phosphate group
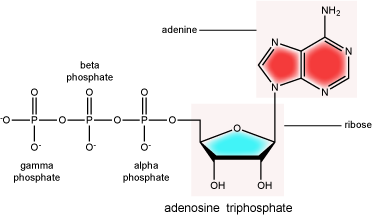
There can be anywhere between one and three phosphate groups bound to the 5' carbon of the sugar. When one phosphate is bound, the nucleotide is referred to as a Nucleotide MonoPhosphate (NMP). If two phosphates are bound the nucleotide is referred to as Nucleotide DiPhosphate (NDP). When three phosphates are bound to the nucleotide it is referred to as a Nucleotide TriPhosphate (NTP). The phosphoanhydride bonds between that link the phosphate groups to each other have specific chemical properties that make them good for various biological functions. The hydrolysis of the bonds between the phosphate groups is thermodynamically exergonic in biological conditions; nature has evolved numerous mechanisms to couple this negative change in free energy to help drive many reactions in the cell. Figure 2 shows the structure of the nucleotide triphosphate Adenosine Triphosphate, ATP, that we will discuss in greater detail in other chapters.
Note: "high-energy" bonds
The term "high-energy bond" is used A LOT in biology. This term is, however, a verbal shortcuts that can cause some confusion. The term refers to the amount of negative free energy associated with the hydrolysis of the bond in question. The water (or other equivalent reaction partner) is an important contributor to the energy calculus. In ATP, for instance, simply "breaking" a phosphoanhydride bond - say with imaginary molecular tweezers - by pulling off a phosphate would not be energetically favorable. We must, therefore, be careful not to say that breaking bonds in ATP is energetically favorable or that it "releases energy". Rather, we should be more specific, noting that they hydrolysis of the bond is energetically favorable. Some of this common misconception is tied to, in our opinion, the use of the term "high energy bonds". While in Bis2a we have tried to minimize the use of the vernacular "high energy" when referring to bonds, trying instead to describe biochemical reactions by using more specific terms, as students of biology you will no doubt encounter the potentially misleading - though admittedly useful - short cut "high energy bond" as you continue in your studies. So, keep the above in mind when you are reading or listening to various discussions in biology. Heck, use the term yourself. Just make sure that you really understand what it refers to.
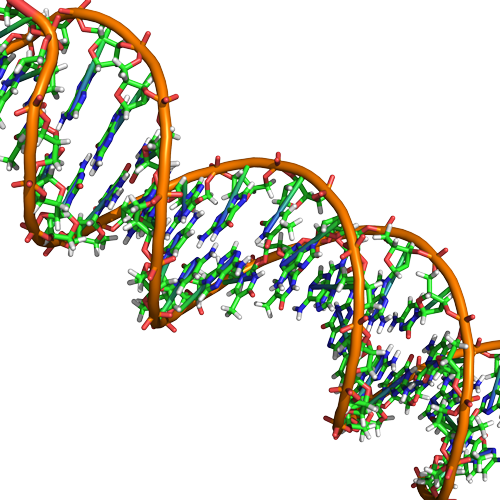
Double helix structure of DNA
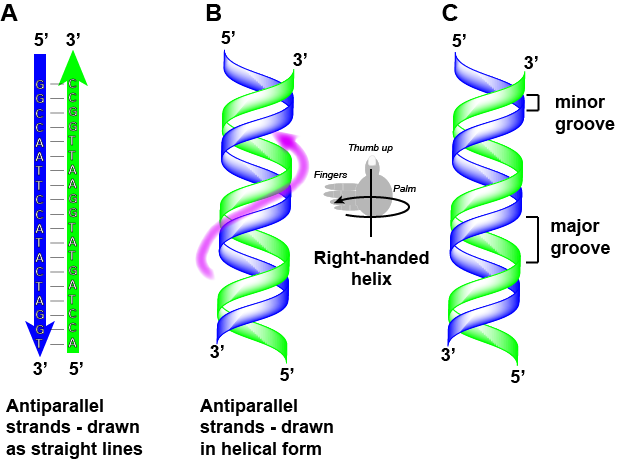
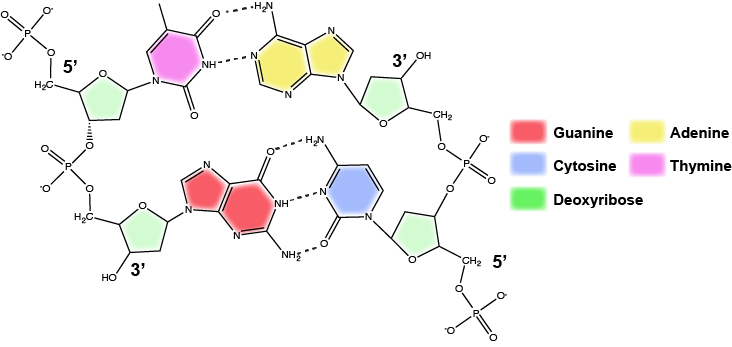
DNA has a double helix structure (shown below) created by two strands of covalently linked nucleotide subunits. The sugar and phosphate groups of each strand of nucleotides are positioned on the outside of the helix, forming the backbone of the DNA (highlighted by the orange ribbons in Figure 3). The two strands of the helix run in opposite directions, meaning that the 5′ carbon end of one strand will face the 3′ carbon end of its matching strand (See Figures 4 and 5). We referred to this orientation of the two strands as antiparallel. Note too that phosphate groups are depicted in Figure 3 as orange and red "sticks" protruding from the ribbon. The phosphates are negatively charged at physiological pHs and therefore give the backbone of the DNA a strong local negatively charged character. By contrast, the nitrogenous bases are stacked in the interior of the helix (these are depicted as green, blue, red, and white sticks in Figure 3). Pairs of nucleotides interact with one another through specific hydrogen bonds (shown in Figure 5). Each pair of separated from the next base pair in the ladder by 0.34 nm and this close stacking and planar orientation gives rise to energetically favorable base-stacking interactions. The specific chemistry associated with these interactions is beyond the content of Bis2a but is described in more detail here for the curious or more advanced students. We do expect, however, that students are aware that the stacking of the nitrogenous bases contributes to the stability of the double helix and defer to your upper-division genetics and organic chemistry instructors to fill in the chemical details.
In a double helix, certain combinations of base pairing are chemically more favored than others based on the types and locations of functional groups on the nitrogenous bases of each nucleotide. In biology we find that:
Adenine (A) is chemically complementary with thymidine (T) (A pairs with T)
and
Guanine (G) is chemically complementary with cytosine (C) (G pairs with C).
We often refer to this pattern as "base complementarity" and say that the antiparallel strands are complementary to each other. For example, if the sequence of one strand is of DNA is 5'-AATTGGCC-3', the complementary strand would have the sequence 5'-GGCCAATT-3'.
We sometimes choose to represent complementary double-helical structures in text by stacking the complementary strands on top of on another as follows:
5' - GGCCAATTCCATACTAGGT - 3'
3' - CCGGTTAAGGTATGATCCA - 5'
Note that each strand has its 5' and 3' ends labeled and that if one were to walk along each strand starting from the 5' end to the 3' end that the direction of travel would be opposite the other for each strand; the strands are antiparallel. We commonly say things like "running 5-prime to 3-prime" or "synthesized 5-prime to 3-prime" to refer to the direction we are reading a sequence or the direction of synthesis. Start getting yourself accustomed to this nomenclature.
Functions and roles of nucleotides and nucleic acids to look out for in Bis2a
In addition to their structural roles in DNA and RNA, nucleotides such as ATP and GTP also serve as mobile energy carriers for the cell. Some students are surprised when they learn to appreciate that the ATP and GTP molecules we discuss in the context of bioenergetics are the same as those involved in the formation of nucleic acids. We will cover this in more detail when we discuss DNA and RNA synthesis reactions. Nucleotides also play important roles as co-factors in many enzymatically catalyzed reactions.
Nucleic acids, RNA in particular, play a variety of roles in in cellular process besides being information storage molecules. Some of the roles that you should keep an eye out for as we progress through the course include: (a) Riboprotein complexes - RNA-Protein complexes in which the RNA serves both catalytic and structural roles. Examples of such complexes include, ribosomes (rRNA), RNases, splicesosome complexes, and telomerase. (b) Information storage and transfer roles. These roles include molecules like DNA, messenger RNA (mRNA), transfer RNA (tRNA). (c) Regulatory roles. Examples of these include various non-coding (ncRNA). Wikipedia has a comprehensive summary of the different types of known RNA molecules that we recommend browsing to get a better sense of the great functional diversity of these molecules.