6: Microbial Metabolism (Part A)
- Page ID
- 77395
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
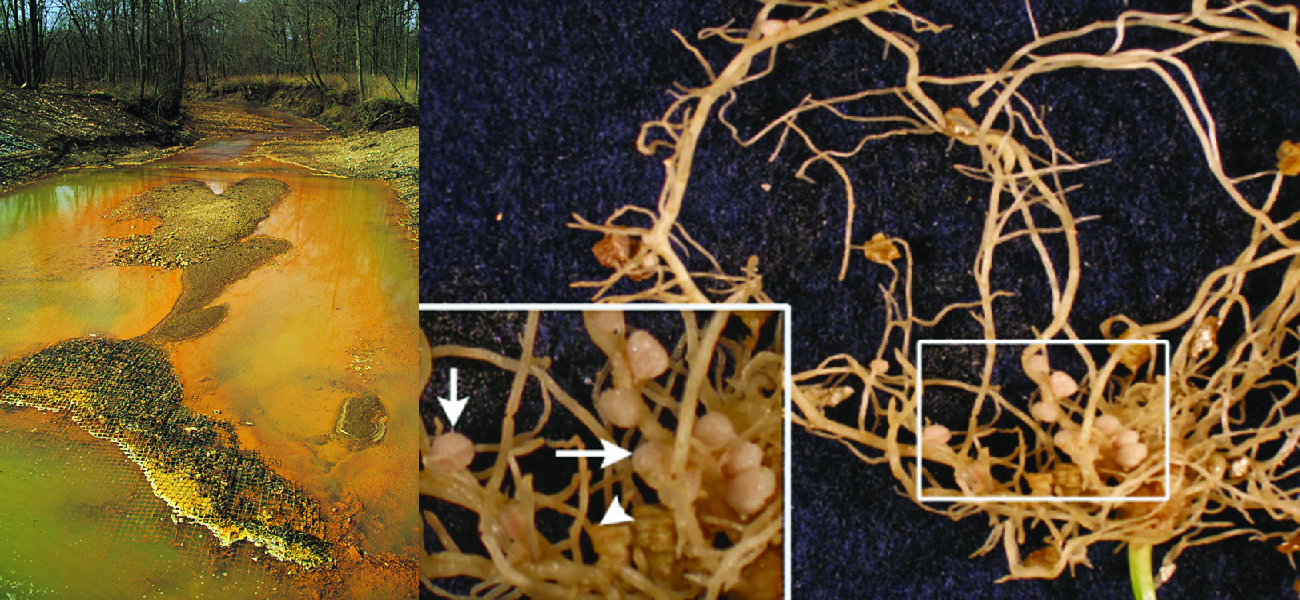
Throughout earth’s history, microbial metabolism has been a driving force behind the development and maintenance of the planet’s biosphere. Eukaryotic organisms such as plants and animals typically depend on organic molecules for energy, growth, and reproduction. Prokaryotes, on the other hand, can metabolize a wide range of organic as well as inorganic matter, from complex organic molecules like cellulose to inorganic molecules and ions such as atmospheric nitrogen (N2), molecular hydrogen (H2), sulfide (S2−), manganese (II) ions (Mn2+), ferrous iron (Fe2+), and ferric iron (Fe3+), to name a few. By metabolizing such substances, microbes chemically convert them to other forms. In some cases, microbial metabolism produces chemicals that can be harmful to other organisms; in others, it produces substances that are essential to the metabolism and survival of other life forms (Figure \(\PageIndex{1}\)).
- 6.1: Energy, Matter, and Enzymes
- Cellular processes such as the building or breaking down of complex molecules occur through series of stepwise, interconnected chemical reactions called metabolic pathways. The term anabolism refers to those endergonic metabolic pathways involved in biosynthesis, converting simple molecular building blocks into more complex molecules, and fueled by the use of cellular energy.
- 6.2: Catabolism of Carbohydrates
- Glycolysis is the first step in the breakdown of glucose, resulting in the formation of ATP, which is produced by substrate-level phosphorylation; NADH; and two pyruvate molecules. Glycolysis does not use oxygen and is not oxygen dependent. After glycolysis, a three-carbon pyruvate is decarboxylated to form a two-carbon acetyl group, coupled with the formation of NADH. The acetyl group is attached to a large carrier compound called coenzyme A.
- 6.3: Cellular Respiration
- Cellular respiration begins when electrons are transferred from NADH and FADH₂—through a series of chemical reactions to a final inorganic electron acceptor (either oxygen in aerobic respiration or non-oxygen inorganic molecules in anaerobic respiration). These electron transfers take place on the inner part of the cell membrane of prokaryotic cells or in specialized protein complexes in the inner membrane of the mitochondria of eukaryotic cells.
Thumbnail: The Krebs cycle, also known as the citric acid cycle, is summarized here. Note incoming two-carbon acetyl results in the main outputs per turn of two CO2, three NADH, one FADH2, and one ATP (or GTP) molecules made by substrate-level phosphorylation. Two turns of the Krebs cycle are required to process all of the carbon from one glucose molecule.


