18.5: Bacterial Infections of the Circulatory and Lymphatic Systems
- Page ID
- 23710
skills to develop
- Identify and compare bacteria that most commonly cause infections of the circulatory and lymphatic systems
- Compare the major characteristics of specific bacterial diseases affecting the circulatory and lymphatic systems
Bacteria can enter the circulatory and lymphatic systems through acute infections or breaches of the skin barrier or mucosa. Breaches may occur through fairly common occurrences, such as insect bites or small wounds. Even the act of tooth brushing, which can cause small ruptures in the gums, may introduce bacteria into the circulatory system. In most cases, the bacteremia that results from such common exposures is transient and remains below the threshold of detection. In severe cases, bacteremia can lead to septicemia with dangerous complications such as toxemia, sepsis, and septic shock. In these situations, it is often the immune response to the infection that results in the clinical signs and symptoms rather than the microbes themselves.
Bacterial Sepsis, Septic and Toxic Shock
At low concentrations, pro-inflammatory cytokines such as interleukin 1 (IL-1) and tumor necrosis factor-α (TNF-α) play important roles in the host’s immune defenses. When they circulate systemically in larger amounts, however, the resulting immune response can be life threatening. IL-1 induces vasodilation (widening of blood vessels) and reduces the tight junctions between vascular endothelial cells, leading to widespread edema. As fluids move out of circulation into tissues, blood pressure begins to drop. If left unchecked, the blood pressure can fall below the level necessary to maintain proper kidney and respiratory functions, a condition known as septic shock. In addition, the excessive release of cytokines during the inflammatory response can lead to the formation of blood clots. The loss of blood pressure and occurrence of blood clots can result in multiple organ failure and death.
Bacteria are the most common pathogens associated with the development of sepsis, and septic shock.1 The most common infection associated with sepsis is bacterial pneumonia (see Bacterial Infections of the Respiratory Tract), accounting for about half of all cases, followed by intra-abdominal infections (Bacterial Infections of the Gastrointestinal Tract) and urinary tract infections (Bacterial Infections of the Urinary System).2 Infections associated with superficial wounds, animal bites, and indwelling catheters may also lead to sepsis and septic shock.
These initially minor, localized infections can be caused by a wide range of different bacteria, including Staphylococcus, Streptococcus, Pseudomonas, Pasteurella, Acinetobacter, and members of the Enterobacteriaceae. However, if left untreated, infections by these gram-positive and gram-negative pathogens can potentially progress to sepsis, shock, and death.
Toxic Shock Syndrome and Streptococcal Toxic Shock-Like Syndrome
Toxemia associated with infections caused by Staphylococcus aureus can cause staphylococcal toxic shock syndrome (TSS). Some strains of S. aureus produce a superantigen called toxic shock syndrome toxin-1 (TSST-1). TSS may occur as a complication of other localized or systemic infections such as pneumonia, osteomyelitis, sinusitis, and skin wounds (surgical, traumatic, or burns). Those at highest risk for staphylococcal TSS are women with preexisting S. aureus colonization of the vagina who leave tampons, contraceptive sponges, diaphragms, or other devices in the vagina for longer than the recommended time.
Staphylococcal TSS is characterized by sudden onset of vomiting, diarrhea, myalgia, body temperature higher than 38.9 °C (102.0 °F), and rapid-onset hypotension with a systolic blood pressure less than 90 mm Hg for adults; a diffuse erythematous rash that leads to peeling and shedding skin 1 to 2 weeks after onset; and additional involvement of three or more organ systems.3 The mortality rate associated with staphylococcal TSS is less than 3% of cases.
Diagnosis of staphylococcal TSS is based on clinical signs, symptoms, serologic tests to confirm bacterial species, and the detection of toxin production from staphylococcal isolates. Cultures of skin and blood are often negative; less than 5% are positive in cases of staphylococcal TSS. Treatment for staphylococcal TSS includes decontamination, debridement, vasopressors to elevate blood pressure, and antibiotic therapy with clindamycin plus vancomycin or daptomycin pending susceptibility results.
A syndrome with signs and symptoms similar to staphylococcal TSS can be caused by Streptococcus pyogenes. This condition, called streptococcal toxic shock-like syndrome (STSS), is characterized by more severe pathophysiology than staphylococcal TSS,4 with about 50% of patients developing S. pyogenes bacteremia and necrotizing fasciitis. In contrast to staphylococcal TSS, STSS is more likely to cause acute respiratory distress syndrome (ARDS), a rapidly progressive disease characterized by fluid accumulation in the lungs that inhibits breathing and causes hypoxemia (low oxygen levels in the blood). STSS is associated with a higher mortality rate (20%–60%), even with aggressive therapy. STSS usually develops in patients with a streptococcal soft-tissue infection such as bacterial cellulitis, necrotizing fasciitis, pyomyositis (pus formation in muscle caused by infection), a recent influenza A infection, or chickenpox.
Exercise \(\PageIndex{1}\)
How can large amounts of pro-inflammatory cytokines lead to septic shock?
Clinical Focus- PART 2
Despite oxacillin therapy, Barbara’s condition continued to worsen over the next several days. Her fever increased to 40.1 °C (104.2 °F) and she began to experience chills, rapid breathing, and confusion. Her doctor suspected bacteremia by a drug-resistant bacterium and admitted Barbara to the hospital. Cultures of the surgical site and blood revealed Staphylococcus aureus. Antibiotic susceptibility testing confirmed that the isolate was methicillin-resistant S. aureus (MRSA). In response, Barbara’s doctor changed her antibiotic therapy to vancomycin and arranged to have the port and venous catheter removed.
Exercise \(\PageIndex{2}\)
- Why did Barbara’s infection not respond to oxacillin therapy?
- Why did the physician have the port and catheter removed?
- Based on the signs and symptoms described, what are some possible diagnoses for Barbara’s condition?
Puerperal Sepsis
A type of sepsis called puerperal sepsis, also known as puerperal infection, puerperal fever, or childbed fever, is a nosocomial infection associated with the period of puerperium—the time following childbirth during which the mother’s reproductive system returns to a nonpregnant state. Such infections may originate in the genital tract, breast, urinary tract, or a surgical wound. Initially the infection may be limited to the uterus or other local site of infection, but it can quickly spread, resulting in peritonitis, septicemia, and death. Before the 19th century work of Ignaz Semmelweis and the widespread acceptance of germ theory (see Modern Foundations of Cell Theory), puerperal sepsis was a major cause of mortality among new mothers in the first few days following childbirth.
Puerperal sepsis is often associated with Streptococcus pyogenes, but numerous other bacteria can also be responsible. Examples include gram-positive bacterial (e.g. Streptococcus spp., Staphylococcus spp., and Enterococcus spp.), gram-negative bacteria (e.g. Chlamydia spp., Escherichia coli, Klebsiella spp., and Proteus spp.), as well as anaerobes such as Peptostreptococcus spp., Bacteroides spp., and Clostridium spp. In cases caused by S. pyogenes, the bacteria attach to host tissues using M protein and produce a carbohydrate capsule to avoid phagocytosis. S. pyogenes also produces a variety of exotoxins, like streptococcal pyrogenic exotoxins A and B, that are associated with virulence and may function as superantigens.
Diagnosis of puerperal fever is based on the timing and extent of fever and isolation, and identification of the etiologic agent in blood, wound, or urine specimens. Because there are numerous possible causes, antimicrobial susceptibility testing must be used to determine the best antibiotic for treatment. Nosocomial incidence of puerperal fever can be greatly reduced through the use of antiseptics during delivery and strict adherence to handwashing protocols by doctors, midwives, and nurses.
Infectious Arthritis
Also called septic arthritis, infectious arthritis can be either an acute or a chronic condition. Infectious arthritis is characterized by inflammation of joint tissues and is most often caused by bacterial pathogens. Most cases of acute infectious arthritis are secondary to bacteremia, with a rapid onset of moderate to severe joint pain and swelling that limits the motion of the affected joint. In adults and young children, the infective pathogen is most often introduced directly through injury, such as a wound or a surgical site, and brought to the joint through the circulatory system. Acute infections may also occur after joint replacement surgery. Acute infectious arthritis often occurs in patients with an immune system impaired by other viral and bacterial infections. S. aureus is the most common cause of acute septic arthritis in the general population of adults and young children. Neisseria gonorrhoeae is an important cause of acute infectious arthritis in sexually active individuals.
Chronic infectious arthritis is responsible for 5% of all infectious arthritis cases and is more likely to occur in patients with other illnesses or conditions. Patients at risk include those who have an HIV infection, a bacterial or fungal infection, prosthetic joints, rheumatoid arthritis (RA), or who are undergoing immunosuppressive chemotherapy. Onset is often in a single joint; there may be little or no pain, aching pain that may be mild, gradual swelling, mild warmth, and minimal or no redness of the joint area.
Diagnosis of infectious arthritis requires the aspiration of a small quantity of synovial fluid from the afflicted joint. Direct microscopic evaluation, culture, antimicrobial susceptibility testing, and polymerase chain reaction (PCR) analyses of the synovial fluid are used to identify the potential pathogen. Typical treatment includes administration of appropriate antimicrobial drugs based on antimicrobial susceptibility testing. For nondrug-resistant bacterial strains, β-lactams such as oxacillin and cefazolin are often prescribed for staphylococcal infections. Third-generation cephalosporins (e.g., ceftriaxone) are used for increasingly prevalent β-lactam-resistant Neisseria infections. Infections by Mycobacterium spp. or fungi are treated with appropriate long-term antimicrobial therapy. Even with treatment, the prognosis is often poor for those infected. About 40% of patients with nongonnococcal infectious arthritis will suffer permanent joint damage and mortality rates range from 5% to 20%.5 Mortality rates are higher among the elderly.6
Osteomyelitis
Osteomyelitis is an inflammation of bone tissues most commonly caused by infection. These infections can either be acute or chronic and can involve a variety of different bacteria. The most common causative agent of osteomyelitis is S. aureus. However, M. tuberculosis, Pseudomonas aeruginosa, Streptococcus pyogenes, S. agalactiae, species in the Enterobacteriaceae, and other microorganisms can also cause osteomyelitis, depending on which bones are involved. In adults, bacteria usually gain direct access to the bone tissues through trauma or a surgical procedure involving prosthetic joints. In children, the bacteria are often introduced from the bloodstream, possibly spreading from focal infections. The long bones, such as the femur, are more commonly affected in children because of the more extensive vascularization of bones in the young.7
The signs and symptoms of osteomyelitis include fever, localized pain, swelling due to edema, and ulcers in soft tissues near the site of infection. The resulting inflammation can lead to tissue damage and bone loss. In addition, the infection may spread to joints, resulting in infectious arthritis, or disseminate into the blood, resulting in sepsis and thrombosis(formation of blood clots). Like septic arthritis, osteomyelitis is usually diagnosed using a combination of radiography, imaging, and identification of bacteria from blood cultures, or from bone cultures if blood cultures are negative. Parenteral antibiotic therapy is typically used to treat osteomyelitis. Because of the number of different possible etiologic agents, however, a variety of drugs might be used. Broad-spectrum antibacterial drugs such as nafcillin, oxacillin, or cephalosporin are typically prescribed for acute osteomyelitis, and ampicillin and piperacillin/tazobactam for chronic osteomyelitis. In cases of antibiotic resistance, vancomycin treatment is sometimes required to control the infection. In serious cases, surgery to remove the site of infection may be required. Other forms of treatment include hyperbaric oxygen therapy (see Using Physical Methods to Control Microorganisms) and implantation of antibiotic beads or pumps.
Exercise \(\PageIndex{3}\)
What bacterium the most common cause of both septic arthritis and osteomyelitis?
Rheumatic Fever
Infections with S. pyogenes have a variety of manifestations and complications generally called sequelae. As mentioned, the bacterium can cause suppurative infections like puerperal fever. However, this microbe can also cause nonsuppurative sequelae in the form of acute rheumatic fever (ARF), which can lead to rheumatic heart disease, thus impacting the circulatory system. Rheumatic fever occurs primarily in children a minimum of 2–3 weeks after an episode of untreated or inadequately treated pharyngitis (see Bacterial Infections of the Respiratory Tract). At one time, rheumatic fever was a major killer of children in the US; today, however, it is rare in the US because of early diagnosis and treatment of streptococcal pharyngitis with antibiotics. In parts of the world where diagnosis and treatment are not readily available, acute rheumatic fever and rheumatic heart disease are still major causes of mortality in children.8
Rheumatic fever is characterized by a variety of diagnostic signs and symptoms caused by nonsuppurative, immune-mediated damage resulting from a cross-reaction between patient antibodies to bacterial surface proteins and similar proteins found on cardiac, neuronal, and synovial tissues. Damage to the nervous tissue or joints, which leads to joint pain and swelling, is reversible. However, damage to heart valves can be irreversible and is worsened by repeated episodes of acute rheumatic fever, particularly during the first 3–5 years after the first rheumatic fever attack. The inflammation of the heart valves caused by cross-reacting antibodies leads to scarring and stiffness of the valve leaflets. This, in turn, produces a characteristic heart murmur. Patients who have previously developed rheumatic fever and who subsequently develop recurrent pharyngitis due to S. pyogenes are at high risk for a recurrent attacks of rheumatic fever.
The American Heart Association recommends9 a treatment regimen consisting of benzathine benzylpenicillin every 3 or 4 weeks, depending on the patient’s risk for reinfection. Additional prophylactic antibiotic treatment may be recommended depending on the age of the patient and risk for reinfection.
Bacterial Endocarditis and Pericarditis
The endocardium is a tissue layer that lines the muscles and valves of the heart. This tissue can become infected by a variety of bacteria, including gram-positive cocci such as Staphylococcus aureus, viridans streptococci, and Enterococcus faecalis, and the gram-negative so-called HACEK bacilli: Haemophilus spp., Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae. The resulting inflammation is called endocarditis, which can be described as either acute or subacute. Causative agents typically enter the bloodstream during accidental or intentional breaches in the normal barrier defenses (e.g., dental procedures, body piercings, catheterization, wounds). Individuals with preexisting heart damage, prosthetic valves and other cardiac devices, and those with a history of rheumatic fever have a higher risk for endocarditis. This disease can rapidly destroy the heart valves and, if untreated, lead to death in just a few days.
In subacute bacterial endocarditis, heart valve damage occurs slowly over a period of months. During this time, blood clots form in the heart, and these protect the bacteria from phagocytes. These patches of tissue-associated bacteria are called vegetations. The resulting damage to the heart, in part resulting from the immune response causing fibrosis of heart valves, can necessitate heart valve replacement (Figure \(\PageIndex{1}\)). Outward signs of subacute endocarditis may include a fever.
Diagnosis of infective endocarditis is determined using the combination of blood cultures, echocardiogram, and clinical symptoms. In both acute and subacute endocarditis, treatment typically involves relatively high doses of intravenous antibiotics as determined by antimicrobial susceptibility testing. Acute endocarditis is often treated with a combination of ampicillin, nafcillin, and gentamicin for synergistic coverage of Staphylococcus spp. and Streptococcus spp. Prosthetic-valve endocarditis is often treated with a combination of vancomycin, rifampin, and gentamicin. Rifampin is necessary to treat individuals with infection of prosthetic valves or other foreign bodies because rifampin can penetrate the biofilm of most of the pathogens that infect these devices.
Staphylcoccus spp. and Streptococcus spp. can also infect and cause inflammation in the tissues surrounding the heart, a condition called acute pericarditis. Pericarditis is marked by chest pain, difficulty breathing, and a dry cough. In most cases, pericarditis is self-limiting and clinical intervention is not necessary. Diagnosis is made with the aid of a chest radiograph, electrocardiogram, echocardiogram, aspirate of pericardial fluid, or biopsy of pericardium. Antibacterial medications may be prescribed for infections associated with pericarditis; however, pericarditis can also be caused other pathogens, including viruses (e.g., echovirus, influenza virus), fungi (e.g., Histoplasma spp., Coccidioides spp.), and eukaryotic parasites (e.g., Toxoplasma spp.).
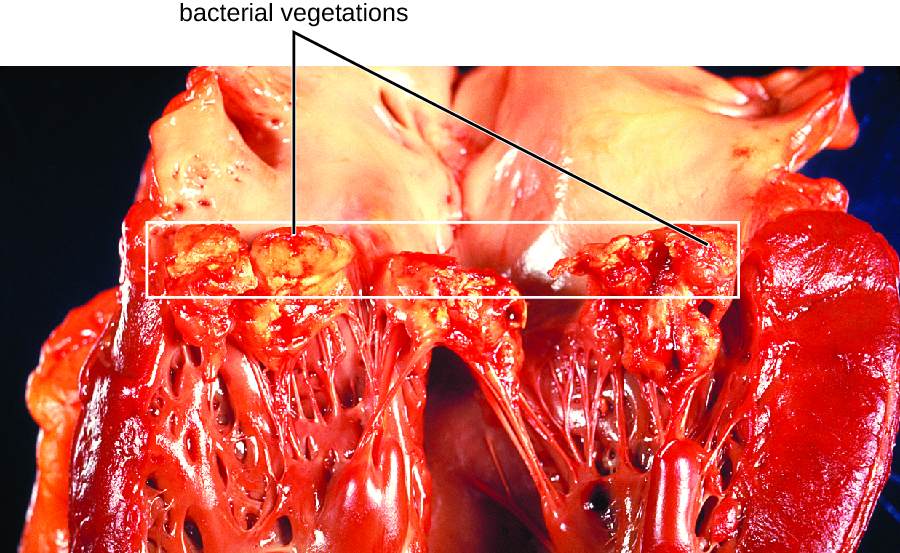
Figure \(\PageIndex{1}\): The heart of an individual who had subacute bacterial endocarditis of the mitral valve. Bacterial vegetations are visible on the valve tissues. (credit: modification of work by Centers for Disease Control and Prevention)
Exercise \(\PageIndex{4}\)
Compare acute and subacute bacterial endocarditis.
Gas Gangrene
Traumatic injuries or certain medical conditions, such as diabetes, can cause damage to blood vessels that interrupts blood flow to a region of the body. When blood flow is interrupted, tissues begin to die, creating an anaerobic environment in which anaerobic bacteria can thrive. This condition is called ischemia. Endospores of the anaerobic bacterium Clostridium perfringens (along with a number of other Clostridium spp. from the gut) can readily germinate in ischemic tissues and colonize the anaerobic tissues.
The resulting infection, called gas gangrene, is characterized by rapidly spreading myonecrosis (death of muscle tissue). The patient experiences a sudden onset of excruciating pain at the infection site and the rapid development of a foul-smelling wound containing gas bubbles and a thin, yellowish discharge tinged with a small amount of blood. As the infection progresses, edema and cutaneous blisters containing bluish-purple fluid form. The infected tissue becomes liquefied and begins sloughing off. The margin between necrotic and healthy tissue often advances several inches per hour even with antibiotic therapy. Septic shock and organ failure frequently accompany gas gangrene; when patients develop sepsis, the mortality rate is greater than 50%.
α-Toxin and theta (θ) toxin are the major virulence factors of C. perfringens implicated in gas gangrene. α-Toxin is a lipase responsible for breaking down cell membranes; it also causes the formation of thrombi (blood clots) in blood vessels, contributing to the spread of ischemia. θ-Toxin forms pores in the patient’s cell membranes, causing cell lysis. The gas associated with gas gangrene is produced by Clostridium’s fermentation of butyric acid, which produces hydrogen and carbon dioxide that are released as the bacteria multiply, forming pockets of gas in tissues (Figure \(\PageIndex{2}\)).
Gas gangrene is initially diagnosed based on the presence of the clinical signs and symptoms described earlier in this section. Diagnosis can be confirmed through Gram stain and anaerobic cultivation of wound exudate (drainage) and tissue samples on blood agar. Treatment typically involves surgical debridement of any necrotic tissue; advanced cases may require amputation. Surgeons may also use vacuum-assisted closure (VAC), a surgical technique in which vacuum-assisted drainage is used to remove blood or serous fluid from a wound or surgical site to speed recovery. The most common antibiotic treatments include penicillin G and clindamycin. Some cases are also treated with hyperbaric oxygen therapy because Clostridium spp. are incapable of surviving in oxygen-rich environments.
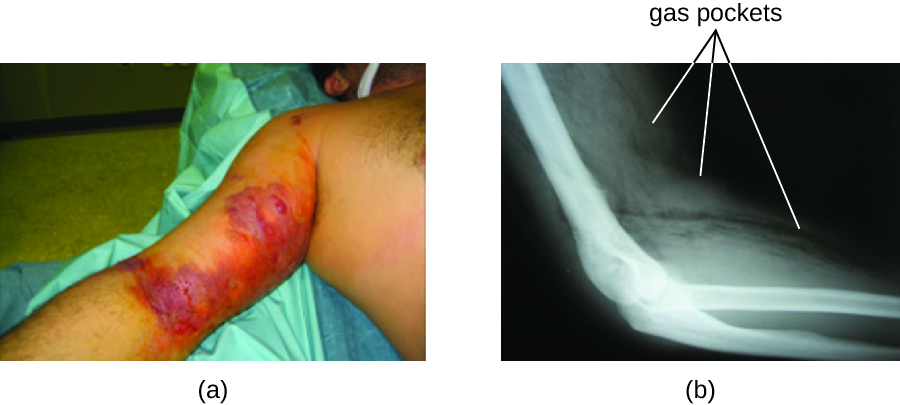
Figure \(\PageIndex{2}\): (a) In this image of a patient with gas gangrene, note the bluish-purple discoloration around the bicep and the irregular margin of the discolored tissue indicating the spread of infection. (b) A radiograph of the arm shows a darkening in the tissue, which indicates the presence of gas. (credit a, b: modification of work by Aggelidakis J, Lasithiotakis K, Topalidou A, Koutroumpas J, Kouvidis G, and Katonis P)
Tularemia
Infection with the gram-negative bacterium Francisella tularensis causes tularemia (or rabbit fever), a zoonotic infection in humans. F. tularensis is a facultative intracellular parasite that primarily causes illness in rabbits, although a wide variety of domesticated animals are also susceptible to infection. Humans can be infected through ingestion of contaminated meat or, more typically, handling of infected animal tissues (e.g., skinning an infected rabbit). Tularemia can also be transmitted by the bites of infected arthropods, including the dog tick (Dermacentor variabilis), the lone star tick (Amblyomma americanum), the wood tick (Dermacentor andersoni), and deer flies (Chrysops spp.). Although the disease is not directly communicable between humans, exposure to aerosols of F. tularensis can result in life-threatening infections. F. tularensis is highly contagious, with an infectious dose of as few as 10 bacterial cells. In addition, pulmonary infections have a 30%–60% fatality rate if untreated.10 For these reasons, F. tularensis is currently classified and must be handled as a biosafety level-3 (BSL-3) organism and as a potential biological warfare agent.
Following introduction through a break in the skin, the bacteria initially move to the lymph nodes, where they are ingested by phagocytes. After escaping from the phagosome, the bacteria grow and multiply intracellularly in the cytoplasm of phagocytes. They can later become disseminated through the blood to other organs such as the liver, lungs, and spleen, where they produce masses of tissue called granulomas (Figure \(\PageIndex{3}\)). After an incubation period of about 3 days, skin lesions develop at the site of infection. Other signs and symptoms include fever, chills, headache, and swollen and painful lymph nodes.
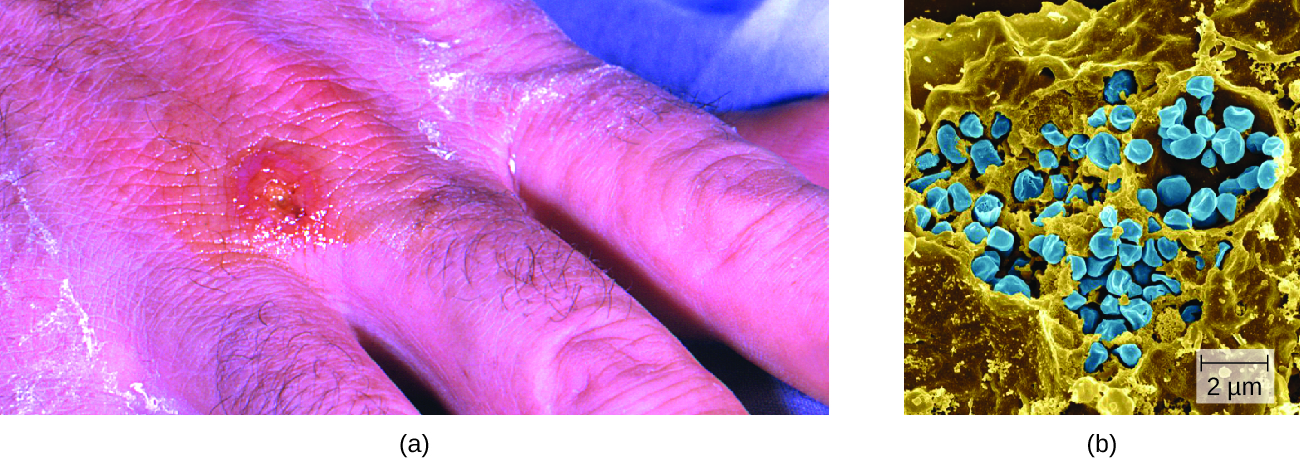
Figure \(\PageIndex{3}\): (a) A skin lesion appears at the site of infection on the hand of an individual infected with Francisella tularensis. (b) A scanning electron micrograph shows the coccobacilli cells (blue) of F. tularensis. (credit a: modification of work by Centers for Disease Control and Prevention; credit b: modification of work by NIAID)
A direct diagnosis of tularemia is challenging because it is so contagious. Once a presumptive diagnosis of tularemia is made, special handling is required to collect and process patients’ specimens to prevent the infection of health-care workers. Specimens suspected of containing F. tularensis can only be handled by BSL-2 or BSL-3 laboratories registered with the Federal Select Agent Program, and individuals handling the specimen must wear protective equipment and use a class II biological safety cabinet.
Tularemia is relatively rare in the US, and its signs and symptoms are similar to a variety of other infections that may need to be ruled out before a diagnosis can be made. Direct fluorescent-antibody (DFA) microscopic examination using antibodies specific for F. tularensis can rapidly confirm the presence of this pathogen. Culturing this microbe is difficult because of its requirement for the amino acid cysteine, which must be supplied as an extra nutrient in culturing media. Serological tests are available to detect an immune response against the bacterial pathogen. In patients with suspected infection, acute- and convalescent-phase serum samples are required to confirm an active infection. PCR-based tests can also be used for clinical identification of direct specimens from body fluids or tissues as well as cultured specimens. In most cases, diagnosis is based on clinical findings and likely incidents of exposure to the bacterium. The antibiotics streptomycin, gentamycin, doxycycline, and ciprofloxacin are effective in treating tularemia.
Brucellosis
Species in the genus Brucella are gram-negative facultative intracellular pathogens that appear as coccobacilli. Several species cause zoonotic infections in animals and humans, four of which have significant human pathogenicity: B. abortus from cattle and buffalo, B. canis from dogs, B. suis from swine, and B. melitensis from goats, sheep, and camels. Infections by these pathogens are called brucellosis, also known as undulant fever, “Mediterranean fever,” or “Malta fever.” Vaccination of animals has made brucellosis a rare disease in the US, but it is still common in the Mediterranean, south and central Asia, Central and South America, and the Caribbean. Human infections are primarily associated with the ingestion of meat or unpasteurized dairy products from infected animals. Infection can also occur through inhalation of bacteria in aerosols when handling animal products, or through direct contact with skin wounds. In the US, most cases of brucellosis are found in individuals with extensive exposure to potentially infected animals (e.g., slaughterhouse workers, veterinarians).
Two important virulence factors produced by Brucella spp. are urease, which allows ingested bacteria to avoid destruction by stomach acid, and lipopolysaccharide (LPS), which allows the bacteria to survive within phagocytes. After gaining entry to tissues, the bacteria are phagocytized by host neutrophils and macrophages. The bacteria then escape from the phagosome and grow within the cytoplasm of the cell. Bacteria phagocytized by macrophages are disseminated throughout the body. This results in the formation of granulomas within many body sites, including bone, liver, spleen, lung, genitourinary tract, brain, heart, eye, and skin. Acute infections can result in undulant (relapsing) fever, but untreated infections develop into chronic disease that usually manifests as acute febrile illness (fever of 40–41 °C [104–105.8 °F]) with recurring flu-like signs and symptoms.
Brucella is only reliably found in the blood during the acute fever stage; it is difficult to diagnose by cultivation. In addition, Brucella is considered a BSL-3 pathogen and is hazardous to handle in the clinical laboratory without protective clothing and at least a class II biological safety cabinet. Agglutination tests are most often used for serodiagnosis. In addition, enzyme-linked immunosorbent assays (ELISAs) are available to determine exposure to the organism. The antibiotics doxycycline or ciprofloxacin are typically prescribed in combination with rifampin; gentamicin, streptomycin, and trimethoprim-sulfamethoxazole (TMP-SMZ) are also effective against Brucella infections and can be used if needed.
Exercise \(\PageIndex{5}\)
Compare the pathogenesis of tularemia and brucellosis.
Cat-Scratch Disease
The zoonosis cat-scratch disease (CSD) (or cat-scratch fever) is a bacterial infection that can be introduced to the lymph nodes when a human is bitten or scratched by a cat. It is caused by the facultative intracellular gram-negative bacterium Bartonella henselae. Cats can become infected from flea feces containing B. henselae that they ingest while grooming. Humans become infected when flea feces or cat saliva (from claws or licking) containing B. henselae are introduced at the site of a bite or scratch. Once introduced into a wound, B. henselae infects red blood cells.
B. henselae invasion of red blood cells is facilitated by adhesins associated with outer membrane proteins and a secretion system that mediates transport of virulence factors into the host cell. Evidence of infection is indicated if a small nodule with pus forms in the location of the scratch 1 to 3 weeks after the initial injury. The bacteria then migrate to the nearest lymph nodes, where they cause swelling and pain. Signs and symptoms may also include fever, chills, and fatigue. Most infections are mild and tend to be self-limiting. However, immunocompromised patients may develop bacillary angiomatosis (BA), characterized by the proliferation of blood vessels, resulting in the formation of tumor-like masses in the skin and internal organs; or bacillary peliosis (BP), characterized by multiple cyst-like, blood-filled cavities in the liver and spleen. Most cases of CSD can be prevented by keeping cats free of fleas and promptly cleaning a cat scratch with soap and warm water.
The diagnosis of CSD is difficult because the bacterium does not grow readily in the laboratory. When necessary, immunofluorescence, serological tests, PCR, and gene sequencing can be performed to identify the bacterial species. Given the limited nature of these infections, antibiotics are not normally prescribed. For immunocompromised patients, rifampin, azithromycin, ciprofloxacin, gentamicin (intramuscularly), or TMP-SMZ are generally the most effective options.
Rat-Bite Fever
The zoonotic infection rat-bite fever can be caused by two different gram-negative bacteria: Streptobacillus moniliformis, which is more common in North America, and Spirillum minor, which is more common in Asia. Because of modern sanitation efforts, rat bites are rare in the US. However, contact with fomites, food, or water contaminated by rat feces or body fluids can also cause infections. Signs and symptoms of rat-bite fever include fever, vomiting, myalgia (muscle pain), arthralgia (joint pain), and a maculopapular rash on the hands and feet. An ulcer may also form at the site of a bite, along with some swelling of nearby lymph nodes. In most cases, the infection is self-limiting. Little is known about the virulence factors that contribute to these signs and symptoms of disease.
Cell culture, MALDI-TOF mass spectrometry, PCR, or ELISA can be used in the identification of Streptobacillus moniliformis. The diagnosis Spirillum minor may be confirmed by direct microscopic observation of the pathogens in blood using Giemsa or Wright stains, or darkfield microscopy. Serological tests can be used to detect a host immune response to the pathogens after about 10 days. The most commonly used antibiotics to treat these infections are penicillin or doxycycline.
Plague
The gram-negative bacillus Yersinia pestis causes the zoonotic infection plague. This bacterium causes acute febrile disease in animals, usually rodents or other small mammals, and humans. The disease is associated with a high mortality rate if left untreated. Historically, Y. pestis has been responsible for several devastating pandemics, resulting in millions of deaths (see Micro Connections: The History of the Plague). There are three forms of plague: bubonic plague(the most common form, accounting for about 80% of cases), pneumonic plague, and septicemic plague. These forms are differentiated by the mode of transmission and the initial site of infection. Figure \(\PageIndex{4}\) illustrates these various modes of transmission and infection between animals and humans.
In bubonic plague, Y. pestis is transferred by the bite of infected fleas. Since most flea bites occur on the legs and ankles, Y. pestis is often introduced into the tissues and blood circulation in the lower extremities. After a 2- to 6-day incubation period, patients experience an abrupt onset fever (39.5–41 °C [103.1–105.8 °F]), headache, hypotension, and chills. The pathogen localizes in lymph nodes, where it causes inflammation, swelling, and hemorrhaging that results in purple buboes (Figure \(\PageIndex{5}\)). Buboes often form in lymph nodes of the groin first because these are the nodes associated with the lower limbs; eventually, through circulation in the blood and lymph, lymph nodes throughout the body become infected and form buboes. The average mortality rate for bubonic plague is about 55% if untreated and about 10% with antibiotic treatment.
Septicemic plague occurs when Y. pestis is directly introduced into the bloodstream through a cut or wound and circulates through the body. The incubation period for septicemic plague is 1 to 3 days, after which patients develop fever, chills, extreme weakness, abdominal pain, and shock. Disseminated intravascular coagulation (DIC) can also occur, resulting in the formation of thrombi that obstruct blood vessels and promote ischemia and necrosis in surrounding tissues (Figure \(\PageIndex{5}\)). Necrosis occurs most commonly in extremities such as fingers and toes, which become blackened. Septicemic plague can quickly lead to death, with a mortality rate near 100% when it is untreated. Even with antibiotic treatment, the mortality rate is about 50%.
Pneumonic plague occurs when Y. pestis causes an infection of the lungs. This can occur through inhalation of aerosolized droplets from an infected individual or when the infection spreads to the lungs from elsewhere in the body in patients with bubonic or septicemic plague. After an incubation period of 1 to 3 days, signs and symptoms include fever, headache, weakness, and a rapidly developing pneumonia with shortness of breath, chest pain, and cough producing bloody or watery mucus. The pneumonia may result in rapid respiratory failure and shock. Pneumonic plague is the only form of plague that can be spread from person to person by infectious aerosol droplet. If untreated, the mortality rate is near 100%; with antibiotic treatment, the mortality rate is about 50%.
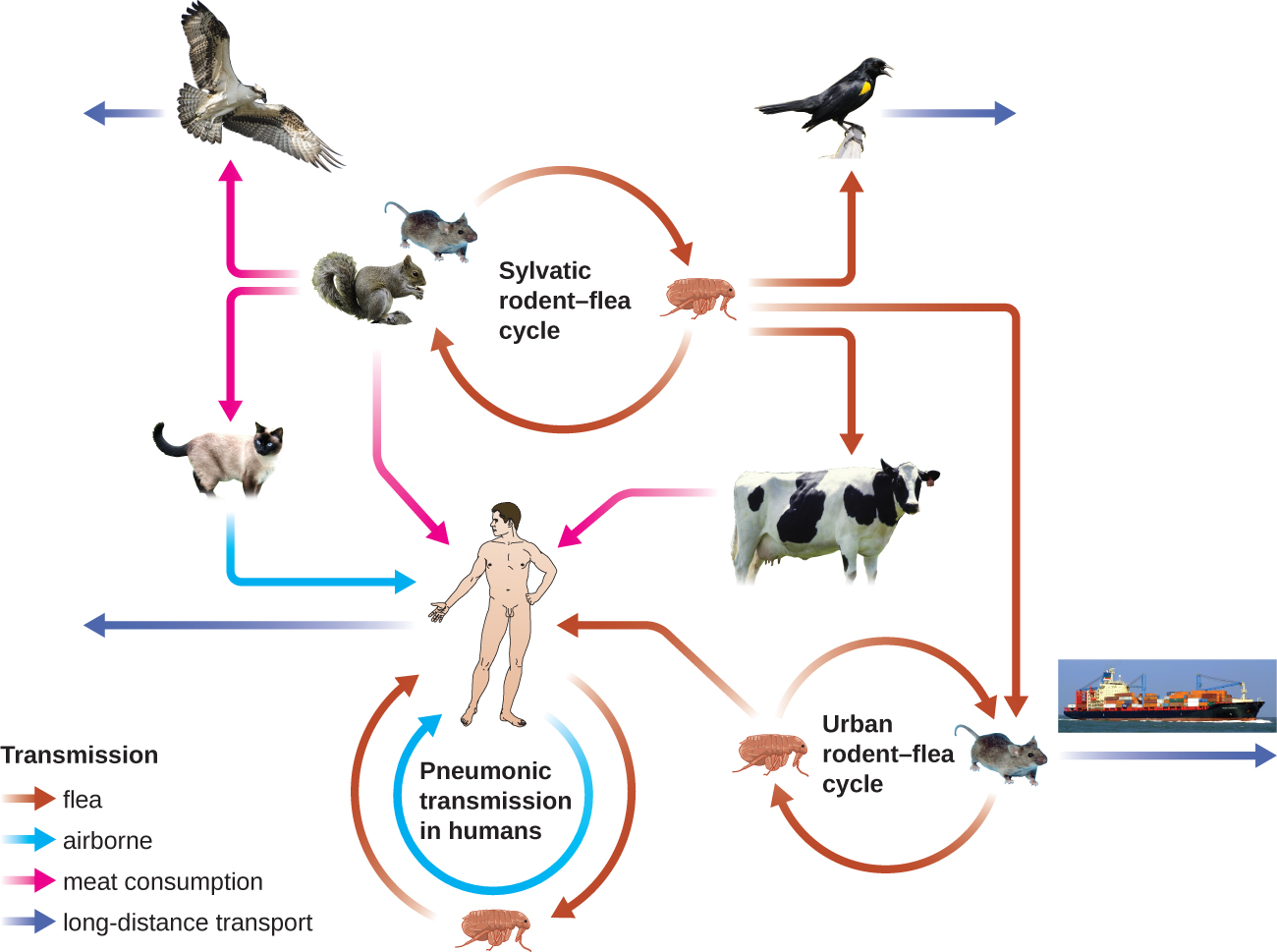
Figure \(\PageIndex{4}\): Yersinia pestis, the causative agent of plague, has numerous modes of transmission. The modes are divided into two ecological classes: urban and sylvatic (i.e., forest or rural). The urban cycle primarily involves transmission from infected urban mammals (rats) to humans by flea vectors (brown arrows). The disease may travel between urban centers (purple arrow) if infected rats find their way onto ships or trains. The sylvatic cycle involves mammals more common in nonurban environments. Sylvatic birds and mammals (including humans) may become infected after eating infected mammals (pink arrows) or by flea vectors. Pneumonic transmission occurs between humans or between humans and infected animals through the inhalation of Y. pestis in aerosols. (credit “diagram”: modification of work by Stenseth NC, Atshabar BB, Begon M, Belmain SR, Bertherat E, Carniel E, Gage KL, Leirs H, and Rahalison L; credit “cat”: modification of work by “KaCey97078”/Flickr)
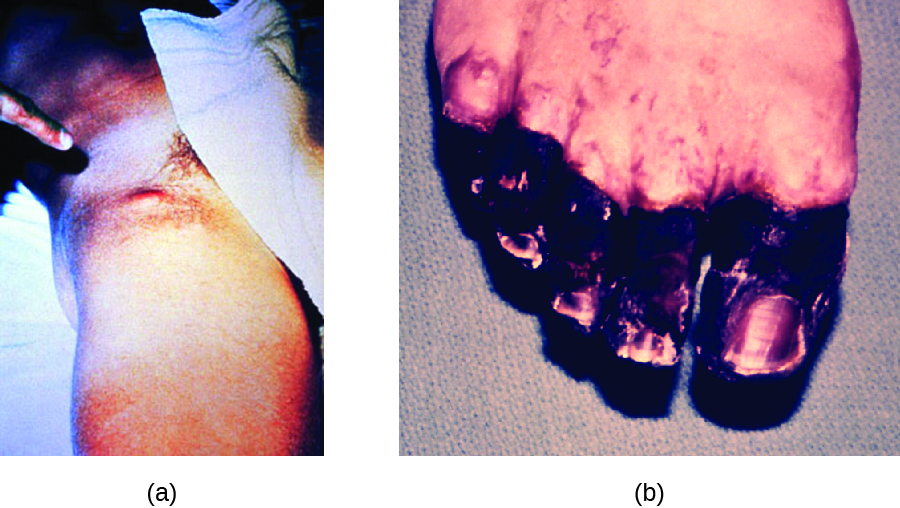
Figure \(\PageIndex{5}\): (a) Yersinia pestis infection can cause inflamed and swollen lymph nodes (buboes), like these in the groin of an infected patient. (b) Septicemic plague caused necrotic toes in this patient. Vascular damage at the extremities causes ischemia and tissue death. (credit a: modification of work by American Society for Microbiology; credit b: modification of work by Centers for Disease Control and Prevention)
The high mortality rate for the plague is, in part, a consequence of it being unusually well equipped with virulence factors. To date, there are at least 15 different major virulence factors that have been identified from Y. pestis and, of these, eight are involved with adherence to host cells. In addition, the F1 component of the Y. pestis capsule is a virulence factor that allows the bacterium to avoid phagocytosis. F1 is produced in large quantities during mammalian infection and is the most immunogenic component.11 Successful use of virulence factors allows the bacilli to disseminate from the area of the bite to regional lymph nodes and eventually the entire blood and lymphatic systems.
Culturing and direct microscopic examination of a sample of fluid from a bubo, blood, or sputum is the best way to identify Y. pestis and confirm a presumptive diagnosis of plague. Specimens may be stained using either a Gram, Giemsa, Wright, or Wayson's staining technique (Figure \(\PageIndex{6}\)). The bacteria show a characteristic bipolar staining pattern, resembling safety pins, that facilitates presumptive identification. Direct fluorescent antibody tests (rapid test of outer-membrane antigens) and serological tests like ELISA can be used to confirm the diagnosis. The confirmatory method for identifying Y. pestis isolates in the US is bacteriophage lysis.
Prompt antibiotic therapy can resolve most cases of bubonic plague, but septicemic and pneumonic plague are more difficult to treat because of their shorter incubation stages. Survival often depends on an early and accurate diagnosis and an appropriate choice of antibiotic therapy. In the US, the most common antibiotics used to treat patients with plague are gentamicin, fluoroquinolones, streptomycin, levofloxacin, ciprofloxacin, and doxycycline.
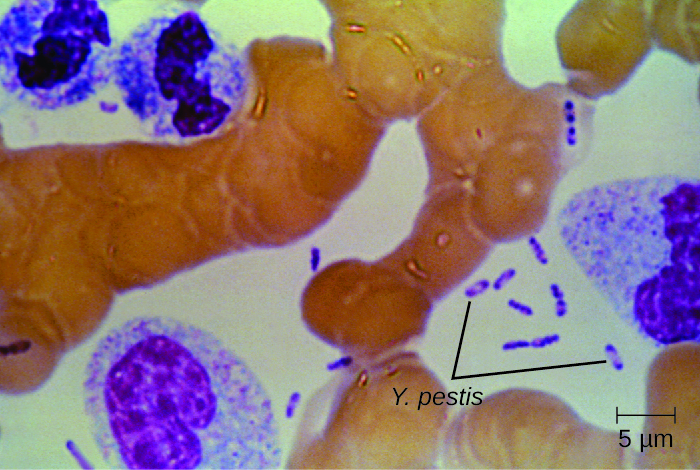
Figure \(\PageIndex{6}\): This Wright’s stain of a blood sample from a patient with plague shows the characteristic “safety pin” appearance of Yersinia pestis. (credit: modification of work by Centers for Disease Control and Prevention)
Exercise \(\PageIndex{6}\)
Compare bubonic plague, septicemic plague, and pneumonic plague.
micro connections - THE HISTORY OF THE PLAGUE
The first recorded pandemic of plague, the Justinian plague, occurred in the sixth century CE. It is thought to have originated in central Africa and spread to the Mediterranean through trade routes. At its peak, more than 5,000 people died per day in Constantinople alone. Ultimately, one-third of that city’s population succumbed to plague.12 The impact of this outbreak probably contributed to the later fall of Emperor Justinian.
The second major pandemic, dubbed the Black Death, occurred during the 14th century. This time, the infections are thought to have originated somewhere in Asia before being transported to Europe by trade, soldiers, and war refugees. This outbreak killed an estimated one-quarter of the population of Europe (25 million, primarily in major cities). In addition, at least another 25 million are thought to have been killed in Asia and Africa.13This second pandemic, associated with strain Yersinia pestis biovar Medievalis, cycled for another 300 years in Europe and Great Britain, and was called the Great Plague in the 1660s.
The most recent pandemic occurred in the 1890s with Yersinia pestis biovar Orientalis. This outbreak originated in the Yunnan province of China and spread worldwide through trade. It is at this time that plague made its way to the US. The etiologic agent of plague was discovered by Alexandre Yersin (1863–1943) during this outbreak as well. The overall number of deaths was lower than in prior outbreaks, perhaps because of improved sanitation and medical support.14 Most of the deaths attributed to this final pandemic occurred in India.
Visit this link to see a video describing how similar the genome of the Black Death bacterium is to today’s strains of bubonic plague.
Zoonotic Febrile Diseases
A wide variety of zoonotic febrile diseases (diseases that cause fever) are caused by pathogenic bacteria that require arthropod vectors. These pathogens are either obligate intracellular species of Anaplasma, Bartonella, Ehrlichia, Orientia, and Rickettsia, or spirochetes in the genus Borrelia. Isolation and identification of pathogens in this group are best performed in BSL-3 laboratories because of the low infective dose associated with the diseases.
Anaplasmosis
The zoonotic tickborne disease human granulocytic anaplasmosis (HGA) is caused by the obligate intracellular pathogen Anaplasma phagocytophilum. HGA is endemic primarily in the central and northeastern US and in countries in Europe and Asia.
HGA is usually a mild febrile disease that causes flu-like symptoms in immunocompetent patients; however, symptoms are severe enough to require hospitalization in at least 50% of infections and, of those patients, less than 1% will die of HGA.15 Small mammals such as white-footed mice, chipmunks, and voles have been identified as reservoirs of A. phagocytophilum, which is transmitted by the bite of an Ixodes tick. Five major virulence factors16 have been reported in Anaplasma; three are adherence factors and two are factors that allow the pathogen to avoid the human immune response. Diagnostic approaches include locating intracellular microcolonies of Anaplasma through microscopic examination of neutrophils or eosinophils stained with Giemsa or Wright stain, PCR for detection of A. phagocytophilum, and serological tests to detect antibody titers against the pathogens. The primary antibiotic used for treatment is doxycycline.
Ehrlichiosis
Human monocytotropic ehrlichiosis (HME) is a zoonotic tickborne disease caused by the BSL-2, obligate intracellular pathogen Ehrlichia chaffeensis. Currently, the geographic distribution of HME is primarily the eastern half of the US, with a few cases reported in the West, which corresponds with the known geographic distribution of the primary vector, the lone star tick (Amblyomma americanum). Symptoms of HME are similar to the flu-like symptoms observed in anaplasmosis, but a rash is more common, with 60% of children and less than 30% of adults developing petechial, macula, and maculopapular rashes.17 Virulence factors allow E. chaffeensis to adhere to and infect monocytes, forming intracellular microcolonies in monocytes that are diagnostic for the HME. Diagnosis of HME can be confirmed with PCR and serologic tests. The first-line treatment for adults and children of all ages with HME is doxycycline.
Epidemic Typhus
The disease epidemic typhus is caused by Rickettsia prowazekii and is transmitted by body lice, Pediculus humanus. Flying squirrels are animal reservoirs of R. prowazekii in North America and can also be sources of lice capable of transmitting the pathogen. Epidemic typhus is characterized by a high fever and body aches that last for about 2 weeks. A rash develops on the abdomen and chest and radiates to the extremities. Severe cases can result in death from shock or damage to heart and brain tissues. Infected humans are an important reservoir for this bacterium because R. prowazekii is the only Rickettsia that can establish a chronic carrier state in humans.
Epidemic typhus has played an important role in human history, causing large outbreaks with high mortality rates during times of war or adversity. During World War I, epidemic typhus killed more than 3 million people on the Eastern front.18With the advent of effective insecticides and improved personal hygiene, epidemic typhus is now quite rare in the US. In the developing world, however, epidemics can lead to mortality rates of up to 40% in the absence of treatment.19 In recent years, most outbreaks have taken place in Burundi, Ethiopia, and Rwanda. For example, an outbreak in Burundi refugee camps in 1997 resulted in 45,000 illnesses in a population of about 760,000 people.20
A rapid diagnosis is difficult because of the similarity of the primary symptoms with those of many other diseases. Molecular and immunohistochemical diagnostic tests are the most useful methods for establishing a diagnosis during the acute stage of illness when therapeutic decisions are critical. PCR to detect distinctive genes from R. prowazekii can be used to confirm the diagnosis of epidemic typhus, along with immunofluorescent staining of tissue biopsy specimens. Serology is usually used to identify rickettsial infections. However, adequate antibody titers take up to 10 days to develop. Antibiotic therapy is typically begun before the diagnosis is complete. The most common drugs used to treat patients with epidemic typhus are doxycycline or chloramphenicol.
Murine (Endemic) Typhus
Murine typhus (also known as endemic typhus) is caused by Rickettsia typhi and is transmitted by the bite of the rat flea, Xenopsylla cheopis, with infected rats as the main reservoir. Clinical signs and symptoms of murine typhusinclude a rash and chills accompanied by headache and fever that last about 12 days. Some patients also exhibit a cough and pneumonia-like symptoms. Severe illness can develop in immunocompromised patients, with seizures, coma, and renal and respiratory failure.
Clinical diagnosis of murine typhus can be confirmed from a biopsy specimen from the rash. Diagnostic tests include indirect immunofluorescent antibody (IFA) staining, PCR for R. typhi, and acute and convalescent serologic testing. Primary treatment is doxycycline, with chloramphenicol as the second choice.
Rocky Mountain Spotted Fever
The disease Rocky Mountain spotted fever (RMSF) is caused by Rickettsia rickettsii and is transmitted by the bite of a hard-bodied tick such as the American dog tick (Dermacentor variabilis), Rocky Mountain wood tick (D. andersoni), or brown dog tick (Rhipicephalus sanguineus).
This disease is endemic in North and South America and its incidence is coincident with the arthropod vector range. Despite its name, most cases in the US do not occur in the Rocky Mountain region but in the Southeast; North Carolina, Oklahoma, Arkansas, Tennessee, and Missouri account for greater than 60% of all cases.21 The map in Figure \(\PageIndex{7}\) shows the distribution of prevalence in the US in 2010.
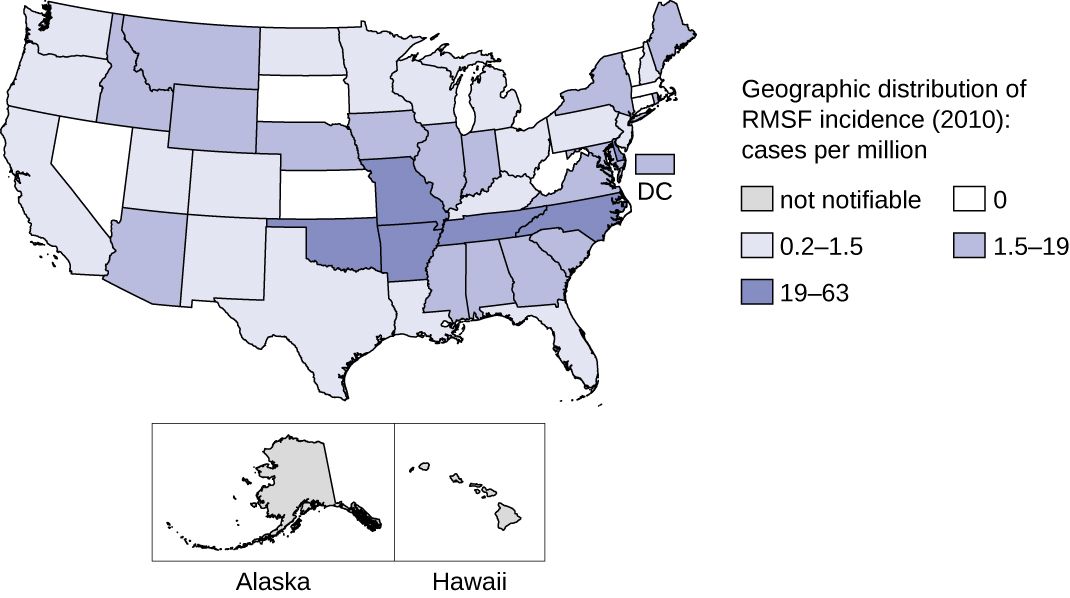
Figure \(\PageIndex{7}\): In the US, Rocky Mountain spotted fever is most prevalent in the southeastern states. (credit: modification of work by Centers for Disease Control and Prevention)
Signs and symptoms of RMSF include a high fever, headache, body aches, nausea, and vomiting. A petechial rash(similar in appearance to measles) begins on the hands and wrists, and spreads to the trunk, face, and extremities (Figure \(\PageIndex{8}\)). If untreated, RMSF is a serious illness that can be fatal in the first 8 days even in otherwise healthy patients. Ideally, treatment should begin before petechiae develop, because this is a sign of progression to severe disease; however, the rash usually does not appear until day 6 or later after onset of symptoms and only occurs in 35%–60% of patients with the infection. Increased vascular permeability associated with petechiae formation can result in fatality rates of 3% or greater, even in the presence of clinical support. Most deaths are due to hypotension and cardiac arrest or from ischemia following blood coagulation.
Diagnosis can be challenging because the disease mimics several other diseases that are more prevalent. The diagnosis of RMSF is made based on symptoms, fluorescent antibody staining of a biopsy specimen from the rash, PCR for Rickettsia rickettsii, and acute and convalescent serologic testing. Primary treatment is doxycycline, with chloramphenicol as the second choice.
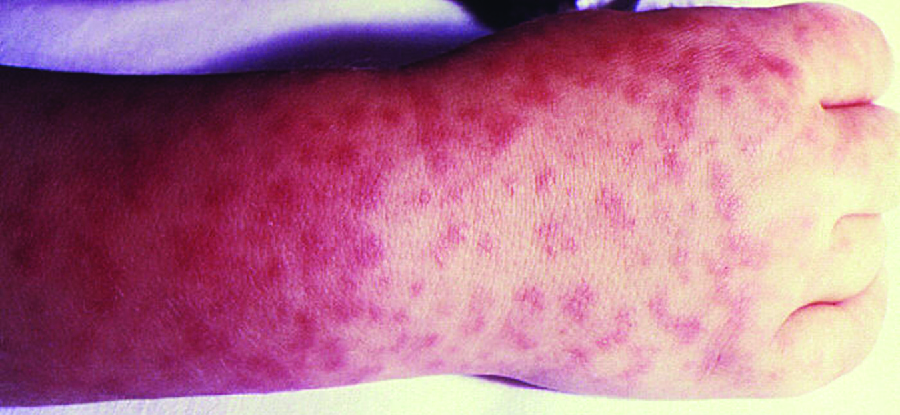
Figure \(\PageIndex{8}\): Rocky Mountain spotted fever causes a petechial rash. Unlike epidemic or murine typhus, the rash begins at the hands and wrists and then spreads to the trunk. (credit: modification of work by Centers for Disease Control and Prevention)
Lyme Disease
Lyme disease is caused by the spirochete Borrelia burgdorferi that is transmitted by the bite of a hard-bodied, black-legged Ixodes tick. I. scapularis is the biological vector transmitting B. burgdorferi in the eastern and north-central US and I. pacificus transmits B. burgdorferi in the western US (Figure \(\PageIndex{10}\)). Different species of Ixodes ticks are responsible for B. burgdorferi transmission in Asia and Europe. In the US, Lyme disease is the most commonly reported vectorborne illness. In 2014, it was the fifth most common Nationally Notifiable disease.22
Ixodes ticks have complex life cycles and deer, mice, and even birds can act as reservoirs. Over 2 years, the ticks pass through four developmental stages and require a blood meal from a host at each stage. In the spring, tick eggs hatch into six-legged larvae. These larvae do not carry B. burgdorferi initially. They may acquire the spirochete when they take their first blood meal (typically from a mouse). The larvae then overwinter and molt into eight-legged nymphs in the following spring. Nymphs take blood meals primarily from small rodents, but may also feed on humans, burrowing into the skin. The feeding period can last several days to a week, and it typically takes 24 hours for an infected nymph to transmit enough B. burgdorferi to cause infection in a human host. Nymphs ultimately mature into male and female adult ticks, which tend to feed on larger animals like deer or, occasionally, humans. The adults then mate and produce eggs to continue the cycle (Figure \(\PageIndex{9}\)).
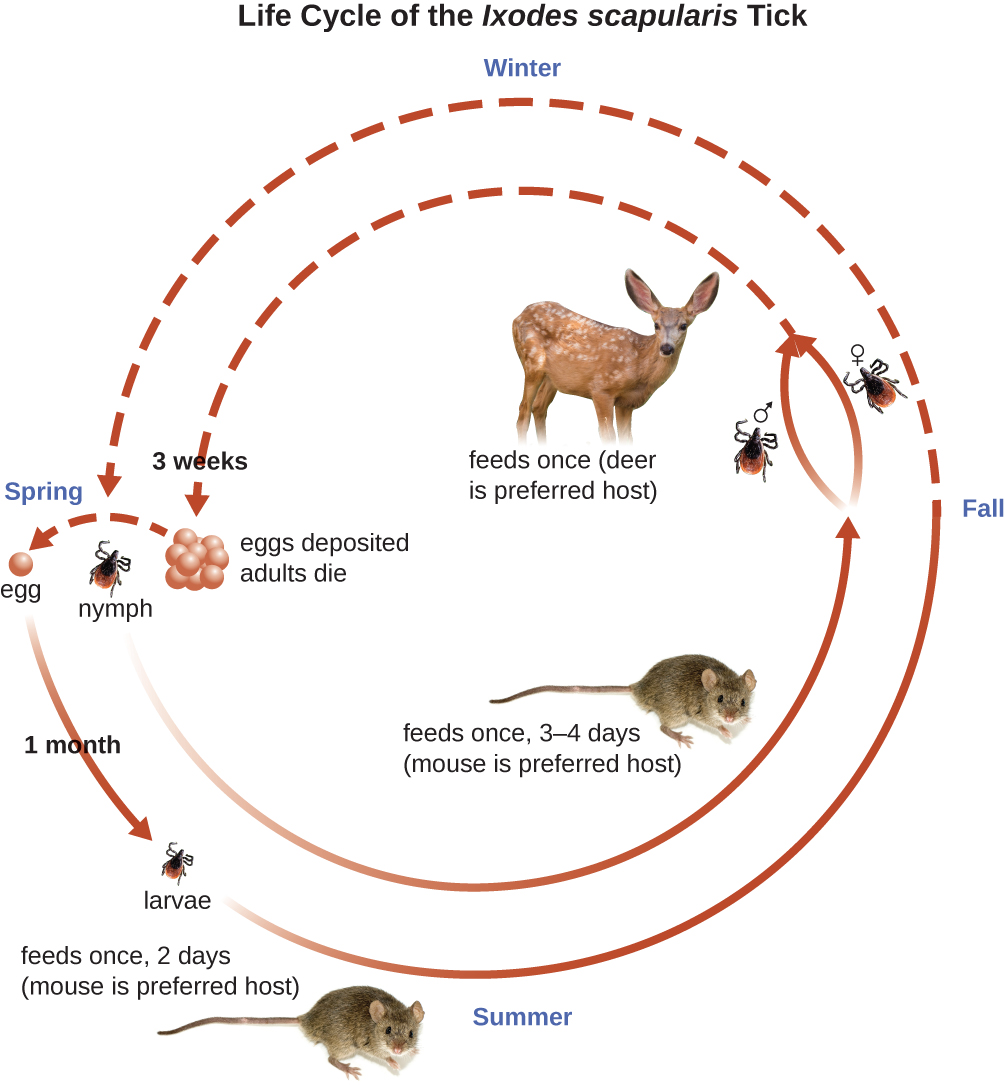
Figure \(\PageIndex{9}\): This image shows the 2-year life cycle of the black-legged tick, the biological vector of Lyme disease. (credit “mouse”: modification of work by George Shuklin)
The symptoms of Lyme disease follow three stages: early localized, early disseminated, and late stage. During the early-localized stage, approximately 70%–80%23 of cases may be characterized by a bull's-eye rash, called erythema migrans, at the site of the initial tick bite. The rash forms 3 to 30 days after the tick bite (7 days is the average) and may also be warm to the touch (Figure \(\PageIndex{10}\)).24 This diagnostic sign is often overlooked if the tick bite occurs on the scalp or another less visible location. Other early symptoms include flu-like symptoms such as malaise, headache, fever, and muscle stiffness. If the patient goes untreated, the second early-disseminated stage of the disease occurs days to weeks later. The symptoms at this stage may include severe headache, neck stiffness, facial paralysis, arthritis, and carditis. The late-stage manifestations of the disease may occur years after exposure. Chronic inflammation causes damage that can eventually cause severe arthritis, meningitis, encephalitis, and altered mental states. The disease may be fatal if untreated.
A presumptive diagnosis of Lyme disease can be made based solely on the presence of a bull’s-eye rash at the site of infection, if it is present, in addition to other associated symptoms (Figure \(\PageIndex{10}\)). In addition, indirect immunofluorescent antibody (IFA) labeling can be used to visualize bacteria from blood or skin biopsy specimens. Serological tests like ELISA can also be used to detect serum antibodies produced in response to infection. During the early stage of infection (about 30 days), antibacterial drugs such as amoxicillin and doxycycline are effective. In the later stages, penicillin G, chloramphenicol, or ceftriaxone can be given intravenously.
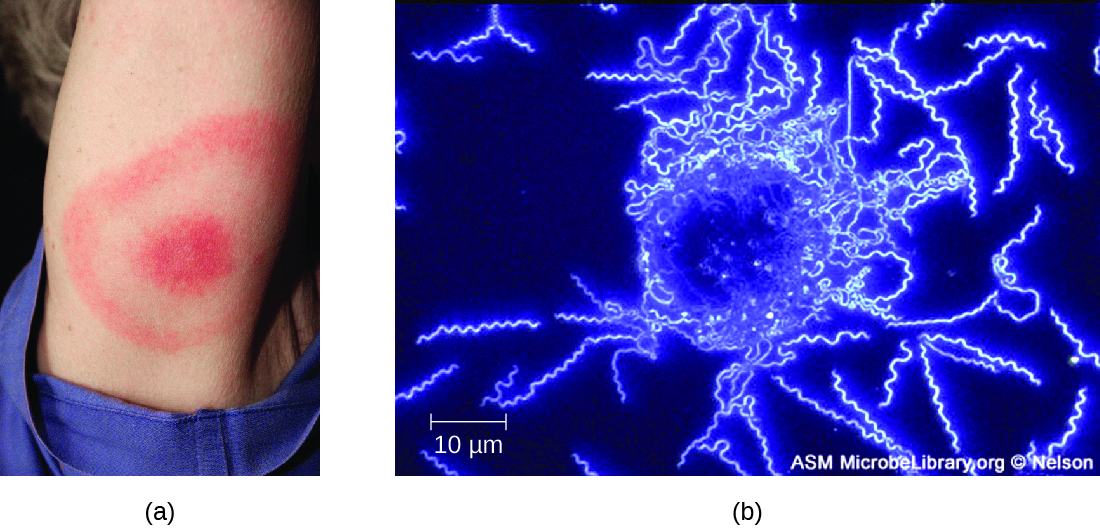
Figure \(\PageIndex{10}\): (a) A characteristic bull’s eye rash of Lyme disease forms at the site of a tick bite. (b) A darkfield micrograph shows Borrelia burgdorferi, the causative agent of Lyme disease. (credit a: modification of work by Centers for Disease Control and Prevention; credit b: modification of work by American Society for Microbiology)
Relapsing Fever
Borrelia spp. also can cause relapsing fever. Two of the most common species are B. recurrentis, which causes epidemics of louseborne relapsing fever, and B. hermsii, which causes tickborne relapsing fevers. These Borrelia species are transmitted by the body louse Pediculus humanus and the soft-bodied tick Ornithodoros hermsi, respectively. Lice acquire the spirochetes from human reservoirs, whereas ticks acquire them from rodent reservoirs. Spirochetes infect humans when Borrelia in the vector’s saliva or excreta enter the skin rapidly as the vector bites.
In both louse- and tickborne relapsing fevers, bacteremia usually occurs after the initial exposure, leading to a sudden high fever (39–43 °C [102.2–109.4 °F) typically accompanied by headache and muscle aches. After about 3 days, these symptoms typically subside, only to return again after about a week. After another 3 days, the symptoms subside again but return a week later, and this cycle may repeat several times unless it is disrupted by antibiotic treatment. Immune evasion through bacterial antigenic variation is responsible for the cyclical nature of the symptoms in these diseases.
The diagnosis of relapsing fever can be made by observation of spirochetes in blood, using darkfield microscopy (Figure \(\PageIndex{11}\)). For louseborne relapsing fever, doxycycline or erythromycin are the first-line antibiotics. For tickborne relapsing fever, tetracycline or erythromycin are the first-line antibiotics.
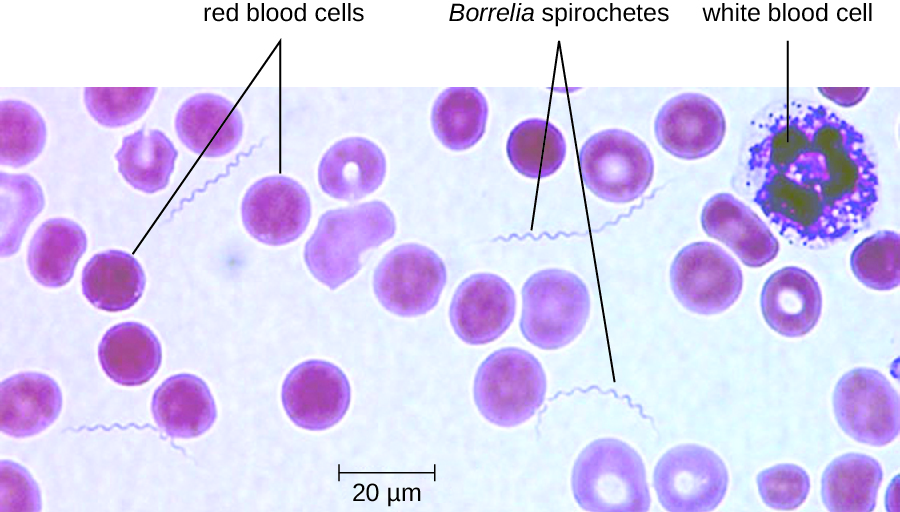
Figure \(\PageIndex{11}\): A peripheral blood smear from a patient with tickborne relapsing fever. Borrelia appears as thin spirochetes among the larger red blood cells. (credit: modification of work by Centers for Disease Control and Prevention)
Trench Fever
The louseborne disease trench fever was first characterized as a specific disease during World War I, when approximately 1 million soldiers were infected. Today, it is primarily limited to areas of the developing world where poor sanitation and hygiene lead to infestations of lice (e.g., overpopulated urban areas and refugee camps). Trench fever is caused by the gram-negative bacterium Bartonella quintana, which is transmitted when feces from infected body lice, Pediculus humanus var corporis, are rubbed into the louse bite, abraded skin, or the conjunctiva. The symptoms typically follow a 5-day course marked by a high fever, body aches, conjunctivitis, ocular pain, severe headaches, and severe bone pain in the shins, neck, and back. Diagnosis can be made using blood cultures; serological tests like ELISA can be used to detect antibody titers to the pathogen and PCR can also be used. The first-line antibiotics are doxycycline, macrolide antibiotics, and ceftriaxone.
Exercise \(\PageIndex{7}\)
- What is the vector associated with epidemic typhus?
- Describe the life cycle of the deer tick and how it spreads Lyme disease.
TICK TIPS
Many of the diseases covered in this chapter involve arthropod vectors. Of these, ticks are probably the most commonly encountered in the US. Adult ticks have eight legs and two body segments, the cephalothorax and the head (Figure \(\PageIndex{12}\)). They typically range from 2 mm to 4 mm in length, and feed on the blood of the host by attaching themselves to the skin.
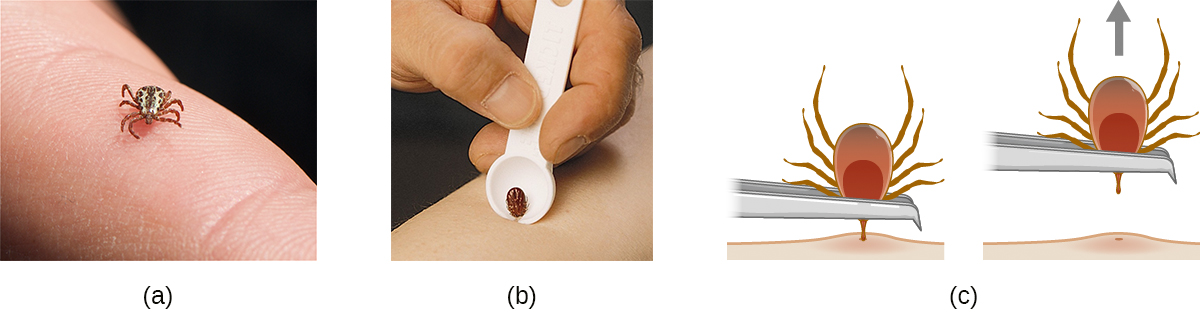
Figure \(\PageIndex{12}\): (a) This black-legged tick, also known as the deer tick, has not yet attached to the skin. (b) A notched tick extractor can be used for removal. (c) To remove an attached tick with fine-tipped tweezers, pull gently on the mouth parts until the tick releases its hold on the skin. Avoid squeezing the tick’s body, because this could release pathogens and thus increase the risk of contracting Lyme disease. (credit a: modification of work by Jerry Kirkhart; credit c: modification of work by Centers for Disease Control and Prevention)
Unattached ticks should be removed and eliminated as soon as they are discovered. When removing a tick that has already attached itself, keep the following guidelines in mind to reduce the chances of exposure to pathogens:
- Use blunt tweezers to gently pull near the site of attachment until the tick releases its hold on the skin.
- Avoid crushing the tick's body and do not handle the tick with bare fingers. This could release bacterial pathogens and actually increase your exposure. The tick can be killed by drowning in water or alcohol, or frozen if it may be needed later for identification and analysis.
- Disinfect the area thoroughly by swabbing with an antiseptic such as isopropanol.
- Monitor the site of the bite for rashes or other signs of infection.
Many ill-advised home remedies for tick removal have become popular in recent years, propagated by social media and pseudojournalism. Health professionals should discourage patients from resorting to any of the following methods, which are NOT recommended:
- using chemicals (e.g., petroleum jelly or fingernail polish) to dislodge an attached tick, because it can cause the tick to release fluid, which can increase the chance of infection
- using hot objects (matches or cigarette butts) to dislodge an attached tick
- squeezing the tick's body with fingers or tweezers
BACTERIAL INFECTIONS OF THE CIRCULATORY AND LYMPHATIC SYSTEMS
Although the circulatory system is a closed system, bacteria can enter the bloodstream through several routes. Wounds, animal bites, or other breaks in the skin and mucous membranes can result in the rapid dissemination of bacterial pathogens throughout the body. Localized infections may also spread to the bloodstream, causing serious and often fatal systemic infections. Figure \(\PageIndex{13}\) and Figure \(\PageIndex{14}\) summarize the major characteristics of bacterial infections of the circulatory and lymphatic systems.
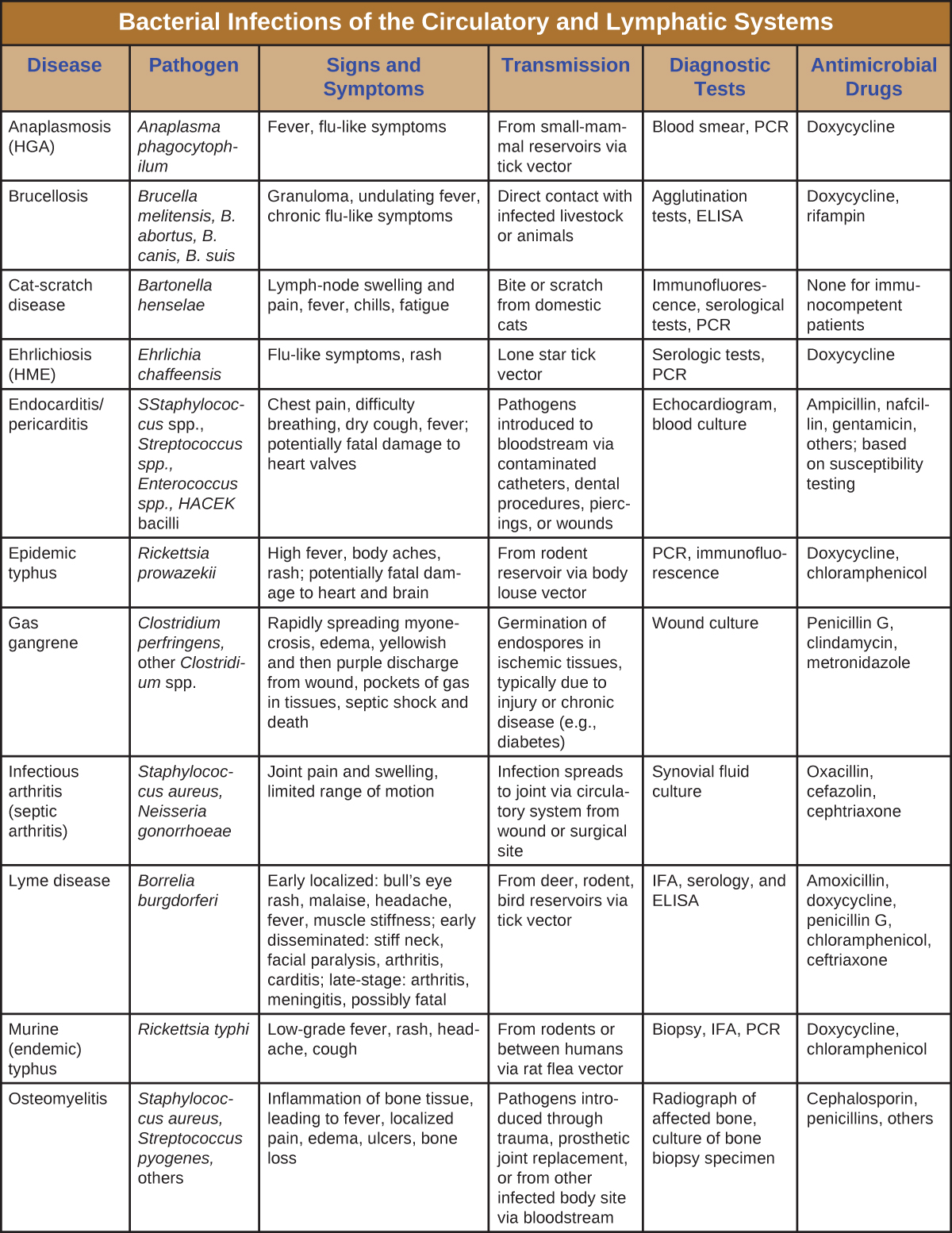
Figure \(\PageIndex{13}\): Bacterial infections of Circulatory and Lymphatic systems.
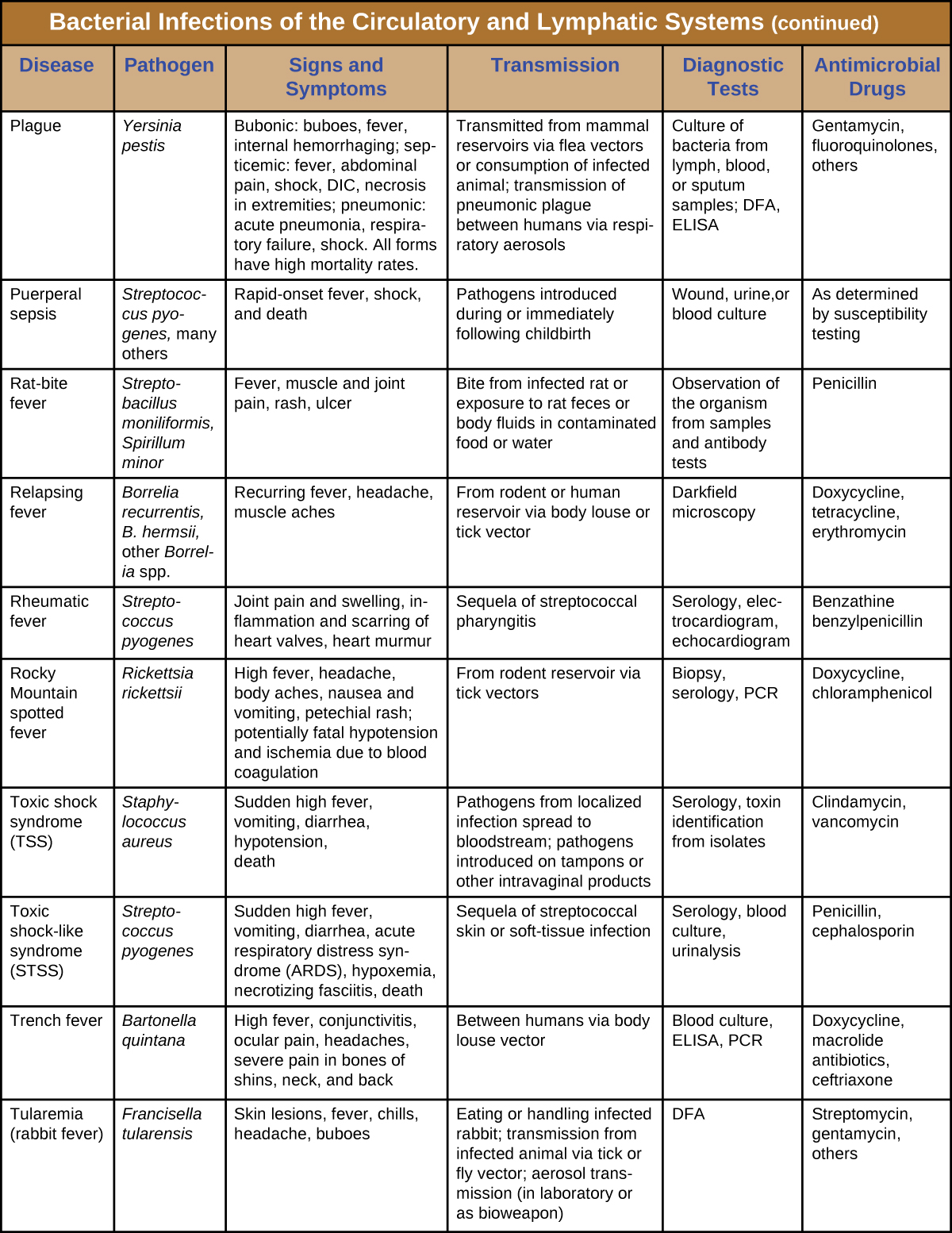
Figure \(\PageIndex{14}\): Bacterial infections of Circulatory and Lymphatic systems.
Key Concepts and Summary
- Bacterial infections of the circulatory system are almost universally serious. Left untreated, most have high mortality rates.
- Bacterial pathogens usually require a breach in the immune defenses to colonize the circulatory system. Most often, this involves a wound or the bite of an arthropod vector, but it can also occur in hospital settings and result in nosocomial infections.
- Sepsis from both gram-negative and gram-positive bacteria, puerperal fever, rheumatic fever, endocarditis, gas gangrene, osteomyelitis, and toxic shock syndrome are typically a result of injury or introduction of bacteria by medical or surgical intervention.
- Tularemia, brucellosis, cat-scratch fever, rat-bite fever, and bubonic plague are zoonotic diseases transmitted by biological vectors
- Ehrlichiosis, anaplasmosis, endemic and murine typhus, Rocky Mountain spotted fever, Lyme disease, relapsing fever, and trench fever are transmitted by arthropod vectors.
- Because their symptoms are so similar to those of other diseases, many bacterial infections of the circulatory system are difficult to diagnose.
- Standard antibiotic therapies are effective for the treatment of most bacterial infections of the circulatory system, unless the bacterium is resistant, in which case synergistic treatment may be required.
- The systemic immune response to a bacteremia, which involves the release of excessive amounts of cytokines, can sometimes be more damaging to the host than the infection itself.
Multiple Choice
Which of the following diseases is caused by a spirochete?
A. tularemia
B. relapsing fever
C. rheumatic fever
D. Rocky Mountain spotted fever
B
Which of the following diseases is transmitted by body lice?
A. tularemia
B. bubonic plague
C. murine typhus
D. epidemic typhus
D
What disease is most associated with Clostridium perfringens?
A. endocarditis
B. osteomyelitis
C. gas gangrene
D. rat bite fever
C
Which bacterial pathogen causes plague?
A. Yersinia pestis
B. Bacillus moniliformis
C. Bartonella quintana
D. Rickettsia rickettsii
A
Fill in the Blank
Lyme disease is characterized by a(n) ________ that forms at the site of infection.
bull’s eye-rash
________ refers to a loss of blood pressure resulting from a system-wide infection.
Septic shock
Short Answer
What are the three forms of plague and how are they contracted?
Compare epidemic and murine typhus.
Critical Thinking
Why are most vascular pathogens poorly communicable from person to person?
How have human behaviors contributed to the spread or control of arthropod-borne vascular diseases?
Footnotes
- 1 S.P. LaRosa. “Sepsis.” 2010. http://www.clevelandclinicmeded.com/...isease/sepsis/.
- 2 D.C. Angus, T. Van der Poll. “Severe Sepsis and Septic Shock.” New England Journal of Medicine 369, no. 9 (2013):840–851.
- 3 Centers for Disease Control and Prevention. “Toxic Shock Syndrome (Other Than Streptococcal) (TSS) 2011 Case Definition.” https://wwwn.cdc.gov/nndss/condition...finition/2011/. Accessed July 25, 2016.
- 4 Centers for Disease Control and Prevention. “Streptococcal Toxic Shock Syndrome (STSS) (Streptococcus pyogenes) 2010 Case Definition.” https://wwwn.cdc.gov/nndss/condition...finition/2010/. Accessed July 25, 2016.
- 5 M.E. Shirtliff, Mader JT. “Acute Septic Arthritis.” Clinical Microbiology Reviews 15 no. 4 (2002):527–544.
- 6 J.R. Maneiro et al. “Predictors of Treatment Failure and Mortality in Native Septic Arthritis.” Clinical Rheumatology 34, no. 11 (2015):1961–1967.
- 7 M. Vazquez. “Osteomyelitis in Children.” Current Opinion in Pediatrics 14, no. 1 (2002):112–115.
- 8 A. Beaudoin et al. “Acute Rheumatic Fever and Rheumatic Heart Disease Among Children—American Samoa, 2011–2012.” Morbidity and Mortality Weekly Report 64 no. 20 (2015):555–558.
- 9 M.A. Gerber et al. “Prevention of Rheumatic Fever and Diagnosis and Treatment of Acute Streptococcal Pharyngitis: A Scientific Statement From the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research: Endorsed by the American Academy of Pediatrics.” Circulation 119, no. 11 (2009):1541–1551.
- 10 World Health Organization. “WHO Guidelines on Tularaemia.” 2007. http://www.cdc.gov/tularemia/resourc...emiamanual.pdf. Accessed July 26, 2016.
- 11 MOH Key Laboratory of Systems Biology of Pathogens. “Virulence Factors of Pathogenic Bacteria, Yersinia.” http://www.mgc.ac.cn/cgi-bin/VFs/gen...Genus=Yersinia. Accessed September 9, 2016.
- 12 Rosen, William. Justinian’s Flea: Plague, Empire, and the Birth of Europe. Viking Adult; pg 3; ISBN 978-0-670-03855-8.
- 13 Benedictow, Ole J. 2004. The Black Death 1346-1353: The Complete History. Woodbridge: Boydell Press.
- 14 Centers for Disease Control and Prevention. “Plague: History.” http://www.cdc.gov/plague/history/. Accessed September 15, 2016.
- 15 J.S. Bakken et al. “Diagnosis and Management of Tickborne Rickettsial Diseases: Rocky Mountain Spotted Fever, Ehrlichioses, and Anaplasmosis–United States. A Practical Guide for Physicians and Other Health Care and Public Health Professionals.” MMWR Recommendations and Reports 55 no. RR04 (2006):1–27.
- 16 MOH Key Laboratory of Systems Biology of Pathogens, “Virulence Factors of Pathogenic Bacteria, Anaplasma” 2016. http://www.mgc.ac.cn/cgi-bin/VFs/jsif/main.cgi. Accessed July, 26, 2016.
- 17 Centers for Disease Control and Prevention. “Ehrlichiosis, Symptoms, Diagnosis, and Treatment.” 2016. https://www.cdc.gov/ehrlichiosis/symptoms/index.html. Accessed July 29, 2016.
- 18 Drali, R., Brouqui, P. and Raoult, D. “Typhus in World War I.” Microbiology Today 41 (2014) 2:58–61.
- 19 Centers for Disease Control and Prevention. CDC Health Information for International Travel 2014: The Yellow Book. Oxford University Press, 2013. http://wwwnc.cdc.gov/travel/yellowbo...s-ehrlichiosis. Accessed July 26, 2016.
- 20 World Health Organization. “Typhus.” 1997. http://www.who.int/mediacentre/factsheets/fs162/en/. Accessed July 26, 2016.
- 21 Centers for Disease Control and Prevention. “Rocky Mountain Spotted Fever (RMSF): Statistics and Epidemiology.” http://www.cdc.gov/rmsf/stats/index.html. Accessed Sept 16, 2016.
- 22 Centers for Disease Control and Prevention. “Lyme Disease. Data and Statistics.” 2015. http://www.cdc.gov/lyme/stats/index.html. Accessed July 26, 2016.
- 23 Centers for Disease Control and Prevention. “Signs and Symptoms of Untreated Lyme Disease.” 2015. http://www.cdc.gov/lyme/signs_symptoms/index.html. Accessed July 27, 2016.
- 24 Centers for Disease Control and Prevention. “Ticks. Symptoms of Tickborne Illness.” 2015. http://www.cdc.gov/ticks/symptoms.html. Accessed July 27, 2016.
Contributor
Nina Parker, (Shenandoah University), Mark Schneegurt (Wichita State University), Anh-Hue Thi Tu (Georgia Southwestern State University), Philip Lister (Central New Mexico Community College), and Brian M. Forster (Saint Joseph’s University) with many contributing authors. Original content via Openstax (CC BY 4.0; Access for free at https://openstax.org/books/microbiology/pages/1-introduction)


