7.1: Introduction to Biochemical Tests Part I
- Page ID
- 52255
Learning Outcomes
- Observe and interpret the fermentation reactions of representative bacteria in phenol red sugar broths, distinguish between respiration and fermentation, discuss the conditions in which these reactions occur.
- Observe and interpret sugar fermentation and hydrogen sulfide formation in TSI agar slants, discuss the purpose of critical ingredients in TSI agar slants, distinguish between different sugar fermentations, interpret TSI reactions.
- Learn about the role of extracellular enzymes in bacteria, observe the hydrolysis of casein hydrolysis
A. Carbohydrate Fermentation
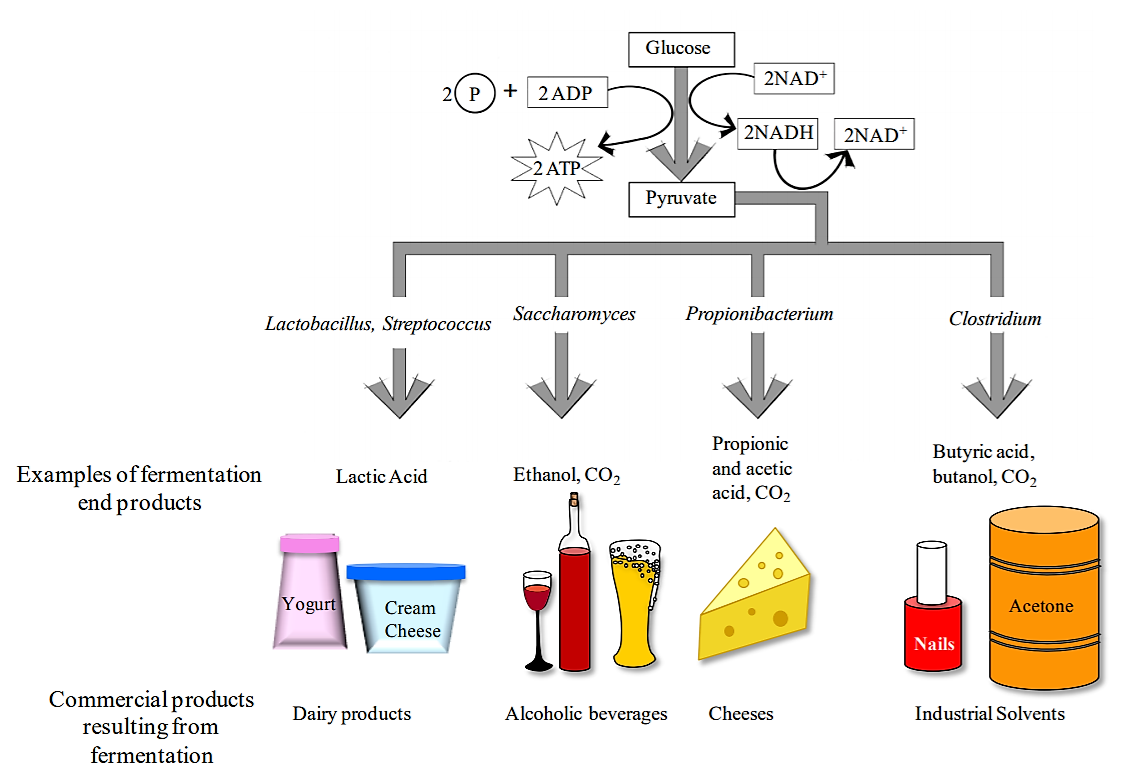
Fermentation is a metabolic process that some microorganisms use to break down substrates such as glucose and other sugars when O2 is not available or could not be used by the microorganism. Fermentation includes the reactions of glycolysis (where a single molecule of glucose is broken down into 2 molecules of pyruvate), as well as additional reactions that produce a variety of end products (acids, alcohols, gases). The end products are characteristic of individual bacterial species. Keep in mind, microbes are very versatile, the fermentation substrate does not have to be sugars, it can include even unusual compounds like aromatics (benzoate), glycerol (sugar-alcohol), and acetylene (hydrocarbons)!
Much of the original energy in the substrate remains tin the chemical bonds of organic end products, like lactic acid or ethanol. For example, one fermentation waste product is ethanol, its got so much stored energy it can be used in gasoline solutions to be combusted/burned to release that energy stored in its chemical bonds.
Note that fermentation is mainly a mechanism for regenerating NAD+ when respiratory process do not occur. Fermentation also tends to produce waste products that can accumulate in the extracellular environment. By contrast, the waste left over after ATP production by aerobic respiration are limited to CO2 and H2O. There can be numerous end products from fermentation, many of which is useful for us, but not necessarily the microbes. We use many fermentation products--as diverse as antibiotics, alcohols, and a variety of foods. Microbes such as yeast and bacteria are genetically engineered to produce valuable fermentation products.
.png?revision=1&size=bestfit&width=859&height=591)
Although the ultimate substrate molecule for fermentation is always glucose, some bacteria use additional chemical reactions to convert other monosaccharides as well as disaccharides into glucose. Therefore bacteria can be differentiated both based on their ability to ferment various carbohydrates, as well as the end products that result from the fermentation process.
The medium used to test carbohydrate fermentation is a nutrient broth that contains a fermentable carbohydrate (usually a monosaccharide or a disaccharide), peptone (amino acids) as well as a pH indicator. The pH of the medium is adjusted to approximately 7.5, so it appears orange/red when using phenol red pH indicator. These types of carbohydrate fermentation tubes are therefore called Phenol red (sugar) broths. If the carbohydrate in the medium is fermented and acidic end products are formed, a color change to yellow will result (see image 1 tubes A and C). Occasionally, bacteria will not ferment the carbohydrate, but instead will break down proteins producing ammonia (NH3) in the growth medium. In this case, the medium will become more alkaline and appear red (see image 1 tube B).
Some bacteria will produce gases when fermenting a carbohydrate. To detect these gases, a Durham tube is used. This is a small inverted glass tube that is placed within the larger glass tube containing the fermentation medium (see image 1). If gases (typically CO2) are produced during the fermentation process, a bubble will form at the top of the Durham tube (see tube A). If you see a bubble in the Durham tube, the medium will also be acidic. Carbohydrate fermentation media are often used to differentiate members of the family Enterobacteriaceae (e.g., Escherichia coli, Enterobacter aerogenes) from each other.
Image 1: Fermentation Reactions Produced by Escherichia coli in Phenol Red Sugar Broths Containing Dextrose, Sucrose, and Lactose sugars. Image by Janie Sigmon, York Technical College, Rock Hill, SC.
In many metabolic tests, end products are produced that change the pH of the medium. To measure this pH change, pH indicators (chemicals that change color depending on pH) are included in the medium. Some common pH indicators are phenol red, bromocresol purple, and bromothymol blue. Each pH indicator has a range of pH values over which it changes color (see below).
| pH Indicator | |||
| phenol red | < pH 6.8 = yellow | pH 6.8 – 7.4 = red | pH >7.4 = pink/magenta |
| bromocresol purple | < pH 6.8 = yellow | > 6.8 pH = purple | |
| bromothymol blue | < pH 6.0 = yellow | pH 6.1 – 7.5 = green | pH >7.5 = blue |
Table 1: pH indicators
Watch Video 1: Phenol red sugar broth tests
Watch Video 1: how to perform phenol red sugar tests. Video by Microbial zoo (3:40). URL: https://youtu.be/W8JWInjlXqQ
B. Triple Sugar Iron (TSI) Agar Slants
Triple Sugar Iron (TSI) agar is a medium used for differentiating enteric bacteria. These bacteria typically reside in the gut/intestines of mammals. Some are major bacterial pathogens, such as certain strains of toxigenic Escherichia coli, Salmonella, Shigella, and Campylobacter species. The TSI medium can differentiate enterics based on their ability to ferment carbohydrates and reduce sulfur. The TSI medium contains three carbohydrates--glucose, lactose, and sucrose-- and iron ions, sodium thiosulfate, and the pH indicator phenol red. The medium is usually made as a 'slant' agar in a glass tube. Bacteria are inoculated into the slant of medium and into the deep portion (called the butt), where it is anaerobic.
There are 3 reactions possible in the TSI agar. First, if it only ferments glucose, then the slant and the butt will turn yellow due to the production of acidic by-products, but after a few hours, the butt remains yellow but the slant itself may will revert back to red as alkaline conditions reappear from the digestion of peptones and the production of ammonium compounds. Second, if lactose or sucrose or both, are fermented, there will be sufficient acid produced to cause both slant and butt to remain yellow. Third, if no carbohydrates are fermented, the slant and butt will remain a red alkaline color.
Gas (CO2) production from carbohydrate fermentation is noted by the presence of cracks or fissures in the medium. If there is a lot of gas, portions of the medium may even be pushed up the tube (Image 2, middle tube/tube 3, notice small gap/space at bottom of tube).
Another thing TSI agar tests is hydrogen sulfide production because it contains the iron ions and sodium thiosulfate. Some bacteria use sodium thiosulfate in their metabolism and release hydrogen sulfide. The hydrogen sulfide reacts with the iron, yielding iron sulfide, which is a black precipitate, the medium will appear black (Image 3 and 4).
Image 2 : Triple sugar iron (TSI) agar was used to grow and differentiate various bacteria. Tube 1 (far left) is the uninoculated control. Tube 2 (second from left) was inoculated with Pseudomonas aeruginosa and displays a red slant with no color change in the butt, indicative of a lack of fermentation. Tube 3 (center) was inoculated with Escherichia coli and displays a yellow slant and a yellow butt, which indicates glucose and lactose and/or sucrose fermentation. It also exhibits cracks in the agar and lifting of the butt, which is indicative of gas production. Tube 4 (second from right) was inoculated with an unidentified culture and displays a red slant and a yellow butt, which indicates that glucose was fermented with acid production. Tube 5 (far right) was inoculated with Gram-positive Staphylococcus aureus and displays a yellow slant and a yellow butt, indicative of glucose and lactose and/or sucrose fermentation. Unlike tube 3, there is no evidence of gas production. All tubes were incubated at 37°C for 24 hours. Image by Clarissa Kaup and J. L. Henriksen, Bellevue University, Bellevue, NE.
Image 3: Proteus mirabilis in a triple sugar iron (TSI) slant. This organism ferments only glucose, indicated by the red coloring of the agar. The slant is red due to depletion of glucose and the subsequent digestion of proteins in the agar. There is a large carbon dioxide bubble in the bottom right area of the tube, and the black precipitate indicates hydrogen sulfide was produced. Image by Diane Hartman, Baylor University, Waco, TX.
Image 4: Proteus vulgaris in a triple sugar iron (TSI) slant. This organism ferments glucose and sucrose. Acid causes the phenol red indicator in the agar to turn yellow. There is a small carbon dioxide bubble in the bottom right area of the tube. The black precipitate indicates hydrogen sulfide was produced. Image by Diane Hartman, Baylor University, Waco, TX.
Image 5: Alcaligenes faecalis in a triple sugar iron (TSI) slant. This organism does not ferment sugars so the medium remains red (no acids are produced in the slant or butt). The slant becomes a deeper shade of red indicating the organism uses the protein in the medium and produces alkaline waste products. There is no carbon dioxide and no hydrogen sulfide (no black precipitate) production. Image by Diane Hartman, Baylor University, Waco, TX.
Watch Video: how to inoculate & interpret TSI agar slants
Watch Video: how to inoculate & interpret a TSI agar slant. Video by MCCC Microbiology (1:35) URL: https://youtu.be/FuOcN3wB0VM
C. Extracellular enzymes
An exoenzyme, or extracellular enzyme, is an enzyme that is secreted by a cell into the environment and functions outside of that cell. Exoenzymes are produced by both prokaryotic and eukaryotic cells. Most often these enzymes are involved in the breakdown of larger macromolecules. The breakdown of these larger macromolecules is critical for allowing their smaller components to pass through the cell membrane and enter into the cell. Bacteria and fungi also produce exoenzymes to digest nutrients in their environment, and these organisms can be used to conduct laboratory assays to identify the presence and function of such exoenzymes. Some pathogenic species also use exoenzymes as virulence factors to assist in their spread.
i. Casein Hydrolysis
Some bacteria secrete extracellular enzymes called proteinases that break down proteins. Milk contains large proteins called casein. Some bacteria secrete caseinases that break down casein outside of the bacterial cell so the smaller products (e.g., amino acids) can be transported inside the cell and further metabolized.
Milk agar (which contains powdered milk) is used to detect the presence of bacterial caseinases. This medium (Image 6) is cloudy because when milk is mixed with agar, the casein forms a colloid through which light cannot pass. The presence of caseinases can be detected by observing a clearing in the agar around the bacterial growth, which indicates that the caseins have been broken down into transparent end products (amino acids and peptides), which are then taken up by the cells (image 7).
Image 6 (left plate): Milk agar contains skim milk (lactose and casein), peptone, and agar. Many organisms can grow on this medium. This medium is used to detect the production of proteases/caseinases that digest casein to soluble peptides. This results in a clear zone. Soluble peptides can then be absorbed by the cell. Casein is responsible for the white color of milk. When digested by exoenzymes, the white agar turns clear and colorless.
Image 7 (right plate): Milk Agar inoculated with (A) Pseudomonas aeruginosa, where casein hydrolysis is indicated by a zone of clearing around the growing colony (green color masking clearing in agar is the diffusable bacterial pigment pyocyanin); (B) Serratia marcescens, where casein hydrolysis is indicated by a zone of clearing around the growing colony (red pigment of bacterium is due to prodigiosin production); (C) Escherichia coli, no casein hydrolysis, notice there is no clearing zone around the culture streak. Images by Tasha Sturm, Cabrillo College, Aptos, CA.
ii. Starch hydrolysis test
Some bacteria produce exoenzymes called hydrolases, which will use water to break apart organic molecules such as the carbohydrate starch. The large polysaccharide molecule starch contains two parts, amylose and amylopectin, these are rapidly hydrolyzed using a hydrolase called alpha-amylase to produce smaller molecules: dextrins, maltose, and glucose. Reaction:
To test for the presence of alpha amylase, a starch hydrolysis test can be performed. Gram's iodine can be used to indicate the presence of starch, when it contacts starch, it forms a blue to brown complex. If the starch has been broken down/hydrolyzed, then there is a clear area that appears in the medium upon addition of Gram's iodine. This clearing zone indicates the presence of alpha amylase.
Image 8: Starch agar incubated for 24 hours at 37°C with Bacillus cereus (left) and Escherichia coli (right). After adding iodine, the iodine binds to starch if it is still present in the agar. A clear zone can be seen around the growth of Bacillus cereus indicating the production of the exoenzyme amylase, which digests starch to glucose leaving nothing behind in the agar for the iodine to bind. Compare his to Escherichia coli, which has no large clearing around the streaked culture area. Image by Tasha Sturm, Cabrillo College, Aptos, CA.
Image 9: Growth of Bacillus subtilis on a starch agar plate before the addition of iodine solution (A) and after the addition of iodine solution (B). After the addition of iodine, the clearing surrounding the bacterial growth indicates starch hydrolysis. Image by Archana Lal, Independence Community College, Independence, KS.
Contributors and Attributions
1. Contributed by Nazzy Pakpour & Sharon Horgan Assistant Professor (Biological Sciences) at California State University
2. Jackie Reynolds, Professor of Biology (Richland College)


