10.9: Transport of Water and Solutes in Plants
- Page ID
- 44666
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Describe how water and solutes are transported in plants
The structure of plant roots, stems, and leaves facilitates the transport of water, nutrients, and photosynthates throughout the plant. The phloem and xylem are the main tissues responsible for this movement. Water potential, evapotranspiration, and stomatal regulation influence how water and nutrients are transported in plants.
Transpiration
Transpiration is the loss of water from the plant through evaporation at the leaf surface. It is the main driver of water movement in the xylem. Transpiration is caused by the evaporation of water at the leaf–atmosphere interface; it creates negative pressure (tension) at the leaf surface. Water from the roots is pulled up by this tension. At night, when stomata shut and transpiration stops, the water is held in the stem and leaf by the adhesion of water to the cell walls of the xylem vessels and tracheids, and the cohesion of water molecules to each other. This is called the cohesion–tension theory of sap ascent.

Inside the leaf at the cellular level, water on the surface of mesophyll cells saturates the cellulose microfibrils of the primary cell wall. The leaf contains many large intercellular air spaces for the exchange of oxygen for carbon dioxide, which is required for photosynthesis. The wet cell wall is exposed to this leaf internal air space, and the water on the surface of the cells evaporates into the air spaces, decreasing the thin film on the surface of the mesophyll cells.
This decrease creates a greater tension on the water in the mesophyll cells (Figure 1), thereby increasing the pull on the water in the xylem vessels. The xylem vessels and tracheids are structurally adapted to cope with large changes in pressure. Rings in the vessels maintain their tubular shape, much like the rings on a vacuum cleaner hose keep the hose open while it is under pressure. Small perforations between vessel elements reduce the number and size of gas bubbles that can form via a process called cavitation. The formation of gas bubbles in xylem interrupts the continuous stream of water from the base to the top of the plant, causing a break termed an embolism in the flow of xylem sap. The taller the tree, the greater the tension forces needed to pull water, and the more cavitation events. In larger trees, the resulting embolisms can plug xylem vessels, making them non-functional. Transpiration is a passive process, meaning that metabolic energy in the form of ATP is not required for water movement. The energy driving transpiration is the difference in energy between the water in the soil and the water in the atmosphere. However, transpiration is tightly controlled.
Control of Transpiration
The atmosphere to which the leaf is exposed drives transpiration, but also causes massive water loss from the plant. Up to 90 percent of the water taken up by roots may be lost through transpiration.
Leaves are covered by a waxy cuticle on the outer surface that prevents the loss of water. Regulation of transpiration, therefore, is achieved primarily through the opening and closing of stomata on the leaf surface. Stomata are surrounded by two specialized cells called guard cells, which open and close in response to environmental cues such as light intensity and quality, leaf water status, and carbon dioxide concentrations. Stomata must open to allow air containing carbon dioxide and oxygen to diffuse into the leaf for photosynthesis and respiration. When stomata are open, however, water vapor is lost to the external environment, increasing the rate of transpiration. Therefore, plants must maintain a balance between efficient photosynthesis and water loss.
Plants have evolved over time to adapt to their local environment and reduce transpiration (Figure 2). Desert plant (xerophytes) and plants that grow on other plants (epiphytes) have limited access to water. Such plants usually have a much thicker waxy cuticle than those growing in more moderate, well-watered environments (mesophytes). Aquatic plants (hydrophytes) also have their own set of anatomical and morphological leaf adaptations.

Xerophytes and epiphytes often have a thick covering of trichomes or of stomata that are sunken below the leaf’s surface. Trichomes are specialized hair-like epidermal cells that secrete oils and substances. These adaptations impede air flow across the stomatal pore and reduce transpiration. Multiple epidermal layers are also commonly found in these types of plants.
Photosynthates
Plants need an energy source to grow. In seeds and bulbs, food is stored in polymers (such as starch) that are converted by metabolic processes into sucrose for newly developing plants. Once green shoots and leaves are growing, plants are able to produce their own food by photosynthesizing. The products of photosynthesis are called photosynthates, which are usually in the form of simple sugars such as sucrose.
Structures that produce photosynthates for the growing plant are referred to as sources. Sugars produced in sources, such as leaves, need to be delivered to growing parts of the plant via the phloem in a process called translocation. The points of sugar delivery, such as roots, young shoots, and developing seeds, are called sinks. Seeds, tubers, and bulbs can be either a source or a sink, depending on the plant’s stage of development and the season.
The products from the source are usually translocated to the nearest sink through the phloem. For example, the highest leaves will send photosynthates upward to the growing shoot tip, whereas lower leaves will direct photosynthates downward to the roots. Intermediate leaves will send products in both directions, unlike the flow in the xylem, which is always unidirectional (soil to leaf to atmosphere). The pattern of photosynthate flow changes as the plant grows and develops. Photosynthates are directed primarily to the roots early on, to shoots and leaves during vegetative growth, and to seeds and fruits during reproductive development. They are also directed to tubers for storage.
Translocation: Transport from Source to Sink
Photosynthates, such as sucrose, are produced in the mesophyll cells of photosynthesizing leaves. From there they are translocated through the phloem to where they are used or stored. Mesophyll cells are connected by cytoplasmic channels called plasmodesmata. Photosynthates move through these channels to reach phloem sieve-tube elements (STEs) in the vascular bundles. From the mesophyll cells, the photosynthates are loaded into the phloem STEs. The sucrose is actively transported against its concentration gradient (a process requiring ATP) into the phloem cells using the electrochemical potential of the proton gradient. This is coupled to the uptake of sucrose with a carrier protein called the sucrose-H+ symporter.
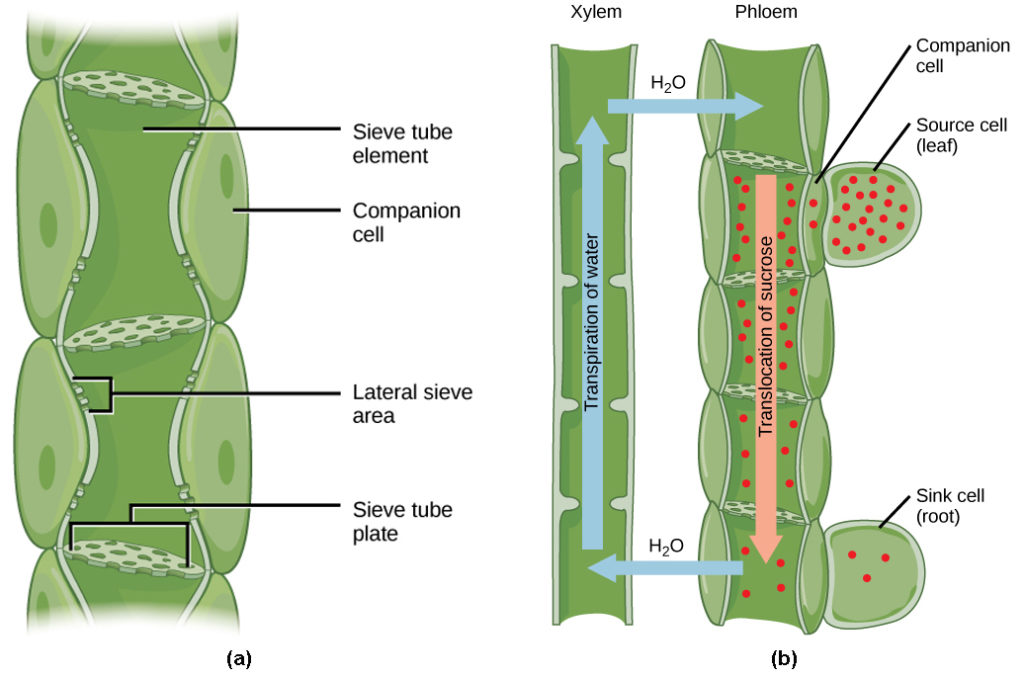
Phloem STEs have reduced cytoplasmic contents, and are connected by a sieve plate with pores that allow for pressure-driven bulk flow, or translocation, of phloem sap. Companion cells are associated with STEs. They assist with metabolic activities and produce energy for the STEs (Figure 3a).
Once in the phloem, the photosynthates are translocated to the closest sink. Phloem sap is an aqueous solution that contains up to 30 percent sugar, minerals, amino acids, and plant growth regulators. This causes the bulk flow of phloem from source to sink (Figure 3b). Sucrose concentration in the sink cells is lower than in the phloem STEs because the sink sucrose has been metabolized for growth, or converted to starch for storage or other polymers, such as cellulose, for structural integrity. Unloading at the sink end of the phloem tube occurs by either diffusion or active transport of sucrose molecules from an area of high concentration to one of low concentration. Water diffuses from the phloem by osmosis and is then transpired or recycled via the xylem back into the phloem sap.
Contributors and Attributions
- Introduction to Transport of Water and Solutes in Plants. Authored by: Shelli Carter and Lumen Learning. Provided by: Lumen Learning. License: CC BY: Attribution
- Biology. Provided by: OpenStax CNX. Located at: http://cnx.org/contents/185cbf87-c72e-48f5-b51e-f14f21b5eabd@10.8. License: CC BY: Attribution. License Terms: Download for free at http://cnx.org/contents/185cbf87-c72...f21b5eabd@10.8

