8.3: Electrons and Energy
- Page ID
- 43609
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Let’s imagine that you are a cell. You’ve just been given a big, juicy glucose molecule, and you’d like to convert some of the energy in this glucose molecule into a more usable form, one that you can use to power your metabolic reactions. How can you go about this? What’s the best way for you to squeeze as much energy as possible out of that glucose molecule, and to capture this energy in a handy form?
Fortunately for us, our cells—and those of other living organisms—are excellent at harvesting energy from glucose and other organic molecules, such as fats and amino acids. Here, we’ll go through a quick overview of how cells break down fuels, then look at the electron transfer reactions (redox reactions) that are key to this process.
Overview of Fuel Breakdown Pathways
The reactions that allow energy to be extracted from molecules such as glucose, fats, and amino acids are called catabolic reactions, meaning that they involve breaking a larger molecule into smaller pieces. For example, when glucose is broken down in the presence of oxygen, it’s converted into six carbon dioxide molecules and six water molecules. The overall reaction for this process can be written as:
This reaction, as written, is simply a combustion reaction, similar to what takes place when you burn a piece of wood in a fireplace or gasoline in an engine. Does this mean that glucose is continually combusting inside of your cells? Thankfully, not quite! The combustion reaction describes the overall process that takes place, but inside of a cell, this process is broken down into many smaller steps. Energy contained in the bonds of glucose is released in small bursts, and some of it can be captured in the form of adenosine triphosphate (ATP), a small molecule that is used to power reactions in the cell. Much of the energy from glucose is still lost as heat, but enough is captured to keep the metabolism of the cell running.
As a glucose molecule is gradually broken down, some of the breakdowns steps release energy that is captured directly as ATP. In these steps, a phosphate group is transferred from a pathway intermediate straight to ADP, a process known as substrate-level phosphorylation. Many more steps, however, produce ATP in an indirect way. In these steps, electrons from glucose are transferred to small molecules known as electron carriers. The electron carriers take the electrons to a group of proteins in the inner membrane of the mitochondrion, called the electron transport chain. As electrons move through the electron transport chain, they go from a higher to a lower energy level and are ultimately passed to oxygen (forming water). Energy released in the electron transport chain is captured as a proton gradient, which powers production of ATP by a membrane protein called ATP synthase. This process is known as oxidative phosphorylation. A simplified diagram of oxidative and substrate-level phosphorylation is shown below.
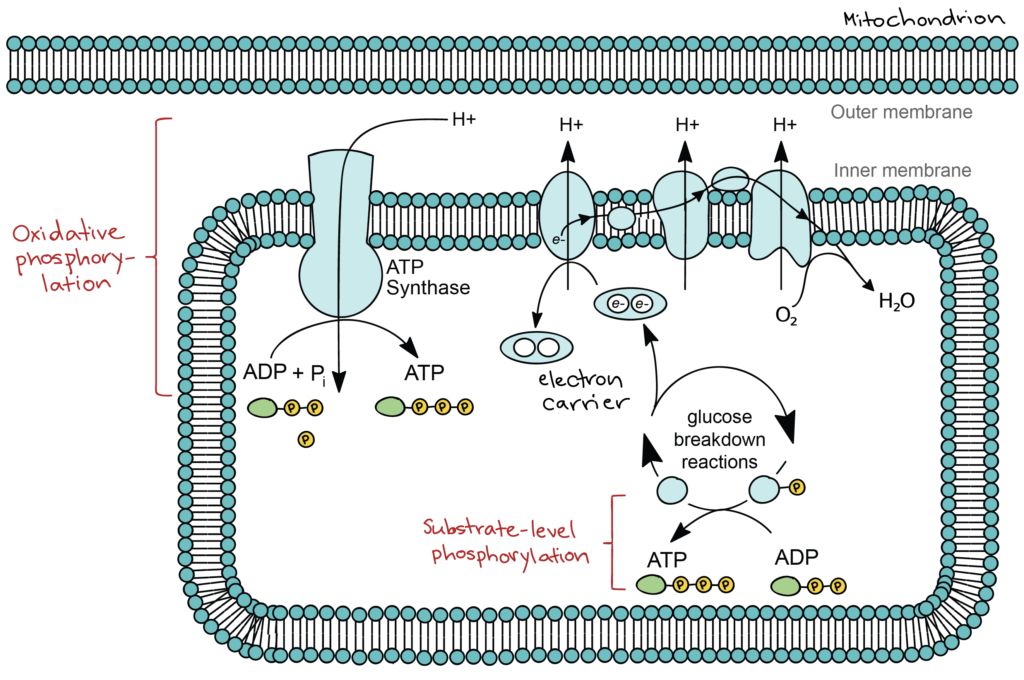
When organic fuels like glucose are broken down using an electron transport chain that ends with oxygen, the breakdown process is known as aerobic respiration (aerobic = oxygen-requiring). Most eukaryotic cells, as well as many bacteria and other prokaryotes, can carry out aerobic respiration. Some prokaryotes have pathways similar to aerobic respiration, but with a different inorganic molecule, such as sulfur, substituted for oxygen. These pathways are not oxygen-dependent, so the breakdown process is called anaerobic respiration (anaerobic = non-oxygen-requiring). Officially, both processes are examples of cellular respiration, the breakdown of down organic fuels using an electron transport chain. However, cellular respiration is commonly used as a synonym for aerobic respiration, and we’ll use it that way here[1].
Redox Reactions
Cellular respiration involves many reactions in which electrons are passed from one molecule to another. Reactions involving electron transfers are known as oxidation-reduction reactions (or redox reactions), and they play a central role in the metabolism of a cell. In a redox reaction, one of the reacting molecules loses electrons and is said to be oxidized, while another reacting molecule gains electrons (the ones lost by the first molecule) and is said to be reduced. You can remember what oxidation and reduction mean with the handy mnemonic “LEO goes GER”: Lose Electrons, Oxidized; Gain Electrons,Reduced. The formation of magnesium chloride is one simple example of a redox reaction:
In this reaction, the magnesium atom loses two electrons, so it is oxidized. These two electrons are accepted by chlorine, which is reduced. The atom or molecule that donates electrons (in this case, magnesium) is called the reducing agent, because its donation of electrons allows another molecule to become reduced. The atom or molecule that accepts the electrons (in this case, chlorine) is known as the oxidizing agent, because its acceptance of electrons allows the other molecule to become oxidized.
Redox Reactions with Carbon-containing Molecules
When a reaction involves the formation of ions, as in the example with magnesium and chlorine above, it’s relatively easy to see that electrons are being transferred. Not all redox reactions involve the complete transfer of electrons, though, and this is particularly true of reactions important in cellular metabolism. Instead, some redox reactions simply change the amount of electron density on a particular atom by altering how it shares electrons in covalent bonds. As an example, let’s consider the combustion of butane:
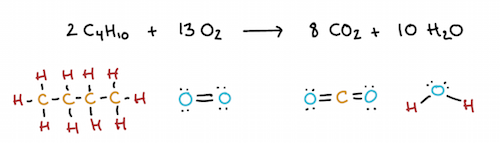
What’s the electron-sharing situation at the start of the reaction? In butane, the carbon atoms are all bonded to other carbons and hydrogens. In bonds, electrons are shared equally, and in
bonds, the
atom has a very slight negative charge (since it’s a bit more electronegative than hydrogen). Similarly, when oxygens are bonded to one another in
, start subscript, 2, end subscript, electrons are shared very equally. After the reaction, however, the electron-sharing picture looks quite different. Oxygen is much more electronegative than carbon, so the in the
bonds of carbon dioxide, oxygen will “hog” the bond electrons. In the
bonds of water, oxygen will similarly pull electrons away from the hydrogen atoms. Thus, relative to its state before the reaction, carbon has lost electron density (because oxygen is now hogging its electrons), while oxygen has gained electron density (because it can now hog electrons shared with other elements). It’s thus reasonable to say that carbon was oxidized during this reaction, while oxygen was reduced. (Hydrogen arguably loses a little electron density too, though its electrons were being hogged to some degree in either case.) Biologists often refer to whole molecules, rather than individual atoms, as being reduced or oxidized; thus, we can say that butane—the source of the carbons—is oxidized, while molecular oxygen—the source of the oxygen atoms—is reduced.
It’s important to understand that oxidation and reduction reactions are fundamentally about the transfer of electrons. In the context of biology, however, you may find it helpful to use the gain or loss of H and O atoms as a proxy for the transfer of electrons. As a general rule of thumb, if a carbon-containing molecule gains H atoms or loses O atoms during a reaction, it’s likely been reduced (gained electrons). Conversely, if it loses H atoms or gains O atoms, it’s probably been oxidized (lost electrons). For example, let’s go back to the reaction for glucose breakdown,. In glucose, carbon is associated with H atoms, while in carbon dioxide, no Hs are present. Thus, we would predict that glucose is oxidized in this reaction.
We can confirm this if we look at the actual electron shifts involved, as in the video below:
Energy in Redox Reactions
Like other chemical reactions, redox reactions involve a free energy change. Reactions that move the system from a higher to a lower energy state are spontaneous and release energy, while those that do the opposite require an input of energy. In redox reactions, energy is released when an electron loses potential energy as a result of the transfer. Electrons have more potential energy when they are associated with less electronegative atoms (such as C or H), and less potential energy when they are associated with a more electronegative atom (such as O). Thus, a redox reaction that moves electrons or electron density from a less to a more electronegative atom will be spontaneous and release energy. For instance, the combustion of butane (above) releases energy because there is a net shift of electron density away from carbon and hydrogen and onto oxygen. If you’ve heard it said that molecules like glucose have “high-energy” electrons, this is a reference to the relatively high potential energy of the electrons in their and
bonds.
Quite a bit of energy can be released when electrons in and
bonds are shifted to oxygen. In a cell, however, it’s not a great idea to release all that energy at once in a combustion reaction. Instead, cells harvest energy from glucose in a controlled fashion, capturing as much of it as possible in the form of ATP. This is accomplished by oxidizing glucose in a gradual, rather than an explosive, sort of way. There are two important ways in which this oxidation is gradual:
- Rather than pulling all the electrons off of glucose at the same time, cellular respiration strips them away in pairs. The redox reactions that remove electron pairs from glucose transfer them to small molecules called electron carriers.
- The electron carriers deposit their electrons in the electron transport chain, a series of proteins and organic molecules in the inner mitochondrial membrane. Electrons are passed from one component to the next in a series of energy-releasing steps, allowing energy to be captured in the form of an electrochemical gradient.
We’ll look at both redox carriers and the electron transport chain in more detail below.
The removal of an electron from a molecule, oxidizing it, results in a decrease in potential energy in the oxidized compound. The electron (sometimes as part of a hydrogen atom), does not remain unbonded, however, in the cytoplasm of a cell. Rather, the electron is shifted to a second compound, reducing the second compound. The shift of an electron from one compound to another removes some potential energy from the first compound (the oxidized compound) and increases the potential energy of the second compound (the reduced compound). The transfer of electrons between molecules is important because most of the energy stored in atoms and used to fuel cell functions is in the form of high-energy electrons. The transfer of energy in the form of electrons allows the cell to transfer and use energy in an incremental fashion—in small packages rather than in a single, destructive burst. This module focuses on the extraction of energy from food; you will see that as you track the path of the transfers, you are tracking the path of electrons moving through metabolic pathways.
Electron Carriers
Electron carriers, sometimes called electron shuttles, are small organic molecules that readily cycle between oxidized and reduced forms and are used to transport electrons during metabolic reactions. There are two electron carriers that play particularly important roles during cellular respiration: NAD+ (nicotinamide adenine dinucleotide, shown below) and FAD (flavin adenine dinucleotide). Both NAD+ and FAD can serve as oxidizing agents, accepting a pair of electrons, along with one or more protons, to switch to their reduced forms. NAD+ accepts two electrons and one H+ to become NADH, while FAD accepts two electrons and two H+ to become FADH2. NAD+ is the primary electron carrier used during cellular respiration, with FAD participating in just one (or two sometimes two) reactions.
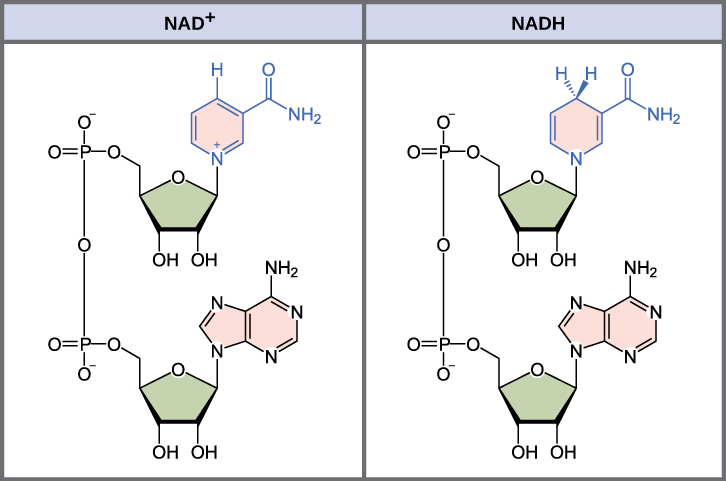
As shown in the image above, NAD+ is a small organic molecule whose structure includes the RNA nucleotide adenine. (FAD is a similar type of molecule, although its functional groups are different.) Both molecules are B vitamin derivatives, with NAD+ produced from niacin and FAD produced from riboflavin. NAD+ and FAD are coenzymes, organic molecules that serve as helpers during enzyme-catalyzed reactions, and they receive electrons and protons as part of these reactions. Specifically, both NAD+ and FAD serve as cofactors for enzymes called dehydrogenases, which remove one or more hydrogen atoms from their substrates.
Electron Transport Chain
In their reduced forms, NADH and FADH2 carry electrons to the electron transport chain in the inner mitochondrial membrane. They deposit their electrons at or near the beginning of the transport chain, and the electrons are then passed along from one protein or organic molecule to the next in a predictable series of steps. Importantly, the movement of electrons through the transport chain is energetically “downhill,” such that energy is released at each step. In redox terms, this means that each member of the electron transport chain is more electronegative (electron-hungry) that the one before it, and less electronegative than the one after[2]. NAD+, which deposits its electrons at the beginning of the chain as NADH, is the least electronegative, while oxygen, which receives the electrons at the end of the chain (along with H+) to form water, is the most electronegative. As electrons trickle “downhill” through the transport chain, they release energy, and some of this energy is captured in the form of an electrochemical gradient and used to make ATP.
- Reece, J. B., Urry, L. A., Cain, M. L., Wasserman, S. A., Minorsky, P. V., and Jackson, R. B. (2011). Cellular respiration and fermentation. In Campbell biology (10th ed., pp. 162–184). San Francisco, CA: Pearson. ↵
- Ibid. ↵
Contributors and Attributions
- Introduction to cellular respiration and redox. Provided by: Khan Academy. Located at: https://www.khanacademy.org/science/biology/cellular-respiration-and-fermentation/intro-to-cellular-respiration/a/intro-to-cellular-respiration-and-redox. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- Oxidation and reduction in cellular respiration. Authored by: Sal Khan. Provided by: Khan Academy. Located at: https://www.khanacademy.org/science/biology/cellular-respiration-and-fermentation/intro-to-cellular-respiration/v/oxidation-and-reduction-in-cellular-respiration. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike



