11: Activity 3-2 - Determining the IC₅₀ of Inhibitor
( \newcommand{\kernel}{\mathrm{null}\,}\)
- Define and distinguish between competitive and noncompetitive inhibition.
- Explain the biochemical meaning of IC₅₀ and Ki, and how they relate to inhibitor potency.
- Predict how changes in substrate or inhibitor concentrations affect enzyme activity.
- Understand the rationale behind choosing substrate and enzyme concentrations for inhibition assays.
- Describe the purpose and logic of a 2-fold serial dilution.
- Enzyme Inhibition: A process in which a molecule decreases enzyme activity by interfering with substrate binding or catalytic function.
- Competitive Inhibitor: A molecule that resembles the substrate and competes for binding at the enzyme's active site.
- Noncompetitive Inhibitor: A molecule that binds to a different site (not the active site) on the enzyme, affecting function regardless of substrate concentration.
- IC₅₀: The concentration of inhibitor that reduces enzyme activity by 50%.
- Ki: The inhibition constant; reflects the binding affinity of an inhibitor to the enzyme.
- P450BM3: A bacterial cytochrome P450 enzyme used in this study for its monooxygenase activity.
- Vmax: The maximum velocity of an enzymatic reaction at saturating substrate levels.
- Km: The substrate concentration at which the reaction rate is half of Vmax.
- Serial Dilution: A stepwise dilution where each step reduces the concentration by a fixed factor (e.g., 2-fold).
- Why is it important to use a substrate concentration slightly above Km in inhibition assays?
- How would you expect the reaction velocity to change with increasing inhibitor concentration?
- What conclusions can you draw if Km increases but Vmax stays constant? What if Vmax decreases and Km remains unchanged?
- Review your prior enzyme kinetics data for P450BM3 (Km and Vmax values).
- Watch a short video or animation on competitive vs. noncompetitive inhibition.
- Familiarize yourself with the spectrophotometer interface if you haven’t used it before.
- Skim through the provided protocol and note any questions about the steps.
Measuring IC₅₀ of Imidazole or 4-Phenylimidazole for P450BM3
Background:
In previous experiments, you examined the kinetic properties of the P450BM3 enzymes, focusing on the determination of Michaelis-Menten constants (Km) and maximum reaction velocities (Vmax). Today’s experiment builds on that foundation by introducing the concept of enzyme inhibition—specifically, how small molecules can interfere with enzyme activity—and how to quantify this effect using IC₅₀ and Ki values. The primary objective is to determine how inhibitors such as imidazole or 4-phenylimidazole affect the activity of the P450BM3 enzyme, a bacterial cytochrome P450 known for its monooxygenase activity.
Enzyme inhibition occurs when a molecule (called an inhibitor) binds to an enzyme and decreases its catalytic activity. One common type is competitive inhibition, in which the inhibitor resembles the substrate and competes for binding at the enzyme’s active site. This type of inhibition is reversible—meaning the inhibitor can bind and unbind—and is highly dependent on substrate concentration. When substrate levels are low, inhibitors can effectively block the active site and reduce enzyme activity. However, at high substrate concentrations, the substrate can "outcompete" the inhibitor for access to the active site, allowing the enzyme to function normally. Because of this, Vmax remains unchanged in competitive inhibition, but Km increases, indicating that a higher substrate concentration is needed to reach half the maximum velocity. This pattern can be visualized in a Lineweaver-Burk plot, where competitive inhibition shows a change in the x-intercept but not in the y-intercept.
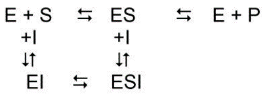
In contrast, noncompetitive inhibition occurs when the inhibitor binds to a site other than the active site. This site can be present on either the free enzyme (E) or the enzyme-substrate complex (ES). Binding at this allosteric site forms an inactive enzyme-inhibitor complex (ESI) that cannot catalyze the reaction. Since the inhibitor does not compete with the substrate directly, increasing the substrate concentration does not reverse the inhibition. In noncompetitive inhibition, Vmax decreases because some enzymes are permanently taken out of the catalytic cycle by the inhibitor. However, Km remains unchanged since the substrate's ability to bind is not directly affected.
In this activity, you will determine the IC₅₀—the concentration of inhibitor that reduces enzyme activity by 50%—for either imidazole or 4-phenylimidazole. These compounds are known inhibitors of P450 enzymes and act by binding to the oxygen-binding heme center, thereby interfering with enzymatic turnover. Based on the data, you will also calculate the Ki (inhibition constant), which reflects the binding affinity of the inhibitor for the enzyme and provides insight into the type and strength of inhibition. Understanding these parameters is crucial for interpreting how small molecules affect enzyme function and for designing selective inhibitors in research or pharmaceutical contexts.


Objective: To determine the inhibitory effect of imidazole and 4-phenylimidazole on the P450BM3 enzyme by calculating IC₅₀ values using spectrophotometric assays.
Materials:
- 100 mM potassium phosphate buffer, pH 7.4 OR 0.5X PBS
- 10 mM lauric acid in 50 mM potassium carbonate (Carolina #871840)
- 10 mM NADPH (Fisher Scientific #481973100MG)
- 100 mM imidazole in 100 mM potassium phosphate OR 0.5X PBS (Fisher Scientific #A1022122)
- 100 µM 4-phenylimidazole in 100 mM potassium phosphate OR 0.5X PBS (Fisher Scientific #L0223704)
- Purified P450BM3 protein (from previous experiments)
- UV/Visible Spectrophotometer (Thermo Scientific #840-300000)
- UVette Cuvettes (Eppendorf #952010051)
- Pipettes and pipette tips
- Incubator or water bath set to 37°C
Procedure: Determine Ki of inhibitors for Kinetics Experiments
Concerns: If you are low on Enzymes volumes, you can decrease your enzyme volume by half or by a 3rd.
- Calculate the volume (in µL) needed to add the following amounts of 100X inhibitor to your cuvettes:
- 16X → ______ µL (Optional)
- 8X → ______ µL
- 4X → ______ µL
- 2X → ______ µL
- 1X → ______ µL
- 0.5X → ______ µL
- 0.25X → ______ µL
- 0.125X → ______ µL (Optional)
- 0.06125X → ______ µL (Optional)
- Set up six (6) cuvettes (or more if you want to do the additional optional steps).
- Follow the protocol from Activities 1-4. However, you will add Enzymes last, instead of NADPH
- You will use the Optimal enzyme concentration volume and Km substrate volume you found in Activities 3.1.
- You will be adjusting the volume of Inhibitors and buffer for each cuvette tested.
- Note: If you are low in enzymes, cut the enzyme volume in half
- Determine the Velocity—Plot Velocity vs. inhibitor concentration to create Michaelis-Menten plots
- Estimate Vmax and Ki. The Ki concentration will be what you will use for the next experiment.
After completing this experiment and analysis, you should be able to:
- Perform a serial dilution and correctly set up a dose-response inhibition assay.
- Accurately measure changes in enzyme activity using spectrophotometric readings.
- Calculate and interpret IC₅₀ values from inhibition curves.
- Differentiate between competitive and noncompetitive inhibition based on kinetic patterns (Vmax and Km changes).
- Use % inhibition data to draw conclusions about inhibitor potency and mechanism.
- Relate your findings to broader applications, such as drug design or enzyme regulation in cells.
- What trends did you observe in your data as the inhibitor concentration increased?
- Based on your IC₅₀ value, which inhibitor appeared more potent: imidazole or 4-phenylimidazole?
- What potential sources of error could have influenced your data (e.g., pipetting, timing, mixing)?
- How would this assay differ if you were testing a noncompetitive inhibitor?
- How do IC₅₀ and Ki relate to real-world drug development?
Data Summary Table (To Complete After Lab)
| Cuvette # | Inhibitor Concentration (µM) | Absorbance Slope | % Inhibition |
|---|---|---|---|
| 1 | |||
| 2 | |||
| … | |||
| 9 |

