1.23: SIM Deep Tests
- Page ID
- 80797
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Explain what a SIM deep is and tell the tests that can be conducted in a SIM deep.
- List possible metabolic reasons why a species of bacteria could be producing hydrogen sulfide.
- Tell what the indole test examines and what enzyme it tests for.
- Explain what the motility is and that is indicates whether or not bacterial species produce flagella for motility.
- Successfully conduct SIM tests and Interpore results of these tests.
SIM Medium
SIM (sulfur reduction, indole, motility) medium is an example combination medium, meaning that one can determine several bacterial activities/characteristics through the use of one medium. SIM medium tests for sulfur reduction, indole production and motility. SIM is an example of a The form of medium used for this test is an agar deep. SIM Medium contains the following: pancreatic digest of casein, peptic digest of animal tissue, ferrous ammonium sulfate Fe(NH4)2(SO4), sodium thiosulfate Na2S2O3, agar (3.5 g/L) and distilled or deionized water.

Figure 1: SIM medium is for deep cultures and an inoculation needle is used to inoculate it. After incubation, a black color indicates that the bacteria has produced hydrogen sulfide (H2S). (Left) This culture turned black and therefore this bacterial species is positive for hydrogen sulfide production. (Right and middle) These cultures did not turn black and is therefore are negative for hydrogen sulfide production. If bacterial are motile, the growth will fan away from the stab. (Right) In this image the bacteria are localized to the stab and therefore no motility occurred and is therefore negative for motility. (Middle) After incubation of a SIM deep, Kovac's reagent may be added to the top of the SIM medium. When Kovac's reagent turns red, this indicates that the bacterial species produced indole and is therefore an indole positive species.
SIM - Sulfur Reduction
Sulfur can be reduced producing hydrogen sulfide (H2S) by bacteria in two unrelated ways:
- One process occurs during putrefaction. When proteins putrefy, the resulting foul “rotten egg” smell is due to the production of hydrogen sulfide gas (H2S). Hydrogen sulfide is a byproduct of the conversion of the amino acid cysteine to pyruvate by the enzyme cysteine desulfurase.
- The second mode of H2S generation involves anaerobic respiration. In some prokaryotes, thiosulfate (S2O32-) is the terminal electron acceptor in an anaerobic respiration. When thiosulfate is reduced (picks up electrons) the result is H2S gas. In either case, invisible H2S gas is produced.
Because hydrogen sulfide gas is colorless (though not odorless!), SIM medium uses an indicator reaction. Iron (in the form of ferrous ammonium sulfate) in the medium combines with H2S gas to form iron sulfide, FeS, a black precipitate. Any black color in the medium indicates the bacterial species is positive for sulfur reduction. If there is no black color in the medium, the bacterial species is negative for sulfur reduction.
Unfortunately, this test does not distinguish between the hydrogen sulfide produced as a result of putrefaction and hydrogen sulfide produced at the end of an anaerobic respiration.

Figure 2: Results of SIM deep cultures (no Kovac's reagent). (Left) The medium is black throughout indicating hydrogen sulfide production and motility. (Middle) The medium is black along the stab line only indicating hydrogen sulfide production and no motility. (Right) The medium is not black and there is no growth away from the stab line indicating this bacterial species is hydrogen sulfide negative and motility negative.
SIM - Indole
Tryptophan is an amino acid found in most proteins. Some bacteria produce tryptophanase, an enzyme that breaks tryptophan down into indole, ammonia and pyruvate (see below). Not all bacterial species produce tryptophanase. Whether a bacterial species produces tryptophanase is dependent on its genes. Testing for the activity of tryptophanase using the indole test is an effective way to differentiate one bacterial species from another and to characterize bacteria.
The pyruvate and ammonia (NH3) are converted into other molecules, but the indole accumulates, and thus can be detected in the media.

Figure 3: Chemical reaction catalyzed by tryptophanase. Bacteria that have the tryptophan gene can break down tryptophan to produce indole. Indole is not used by the bacteria and therefore will accumulate in the medium. Kovac's reagent can be used to detect the accumulated indole and therefore is an effective way of determining if the bacterial species produces the enzyme tryptophanase. Pyruvate is utilized by bacteria in either the cellular respiration metabolic pathway or in a fermentation pathway.
The presence of indole therefore indicates that an organism produces the enzyme tryptophanase. Indole can be detected using a chemical known as Kovac’s reagent. Indole forms a red ring with the addition of Kovac’s reagent indicating the bacterial species is indole positive and that it produces tryptophanase. When a bacterial species is indole negative (indicating no tryptophanase activity), the Kovac's reagent will produce a dark yellow ring.

Figure 4: Results from indole test. Kovac's reagent is on top of the SIM agar deep inside of these test tubes. The reagent appears yellow when the bacterial species is indole negative (left) and the reagent appears red when the bacterial species is indole positive (right).

Figure 5: Reaction that occurs between indole and Kovac's reagent. A reaction between indole and Kovac's reagent produces a dye that is pink-red when indole is present.
SIM - Motility
Motility is the ability of a microbe to “swim” using flagella. The reduced agar content of this medium, 3.5 g/L compared to 12-15 g/L in most solid media, creates a semi liquid environment allowing motile cells to spread from their original placement. The stab technique deposits cells in a straight line down the center of the deep using an inoculation needle rather than an inoculation loop. If growth is observed beyond the stab line into the periphery of the tube, the test is positive for motility. Avoid confusing growth produced by the lateral movement of the needle during an imperfect stab inoculation with actual motility. Rotating the tube for a side view will help you determine if growth is confined to the original inoculation line or has truly spread into the periphery of the tube.

Figure 6: How to inoculate a deep with an inoculation needle. Hold the inoculation needle vertically and stab straight down the center of the deep. Then remove the inoculation needle along the original stab line.
Motility is indicated by the ability of the organism to ‘fan’ away from the streak. Or, the entire tube may appear cloudy when compared to an un-inoculated control. If the organism is non-motile, the growth will only appear along the stab line.
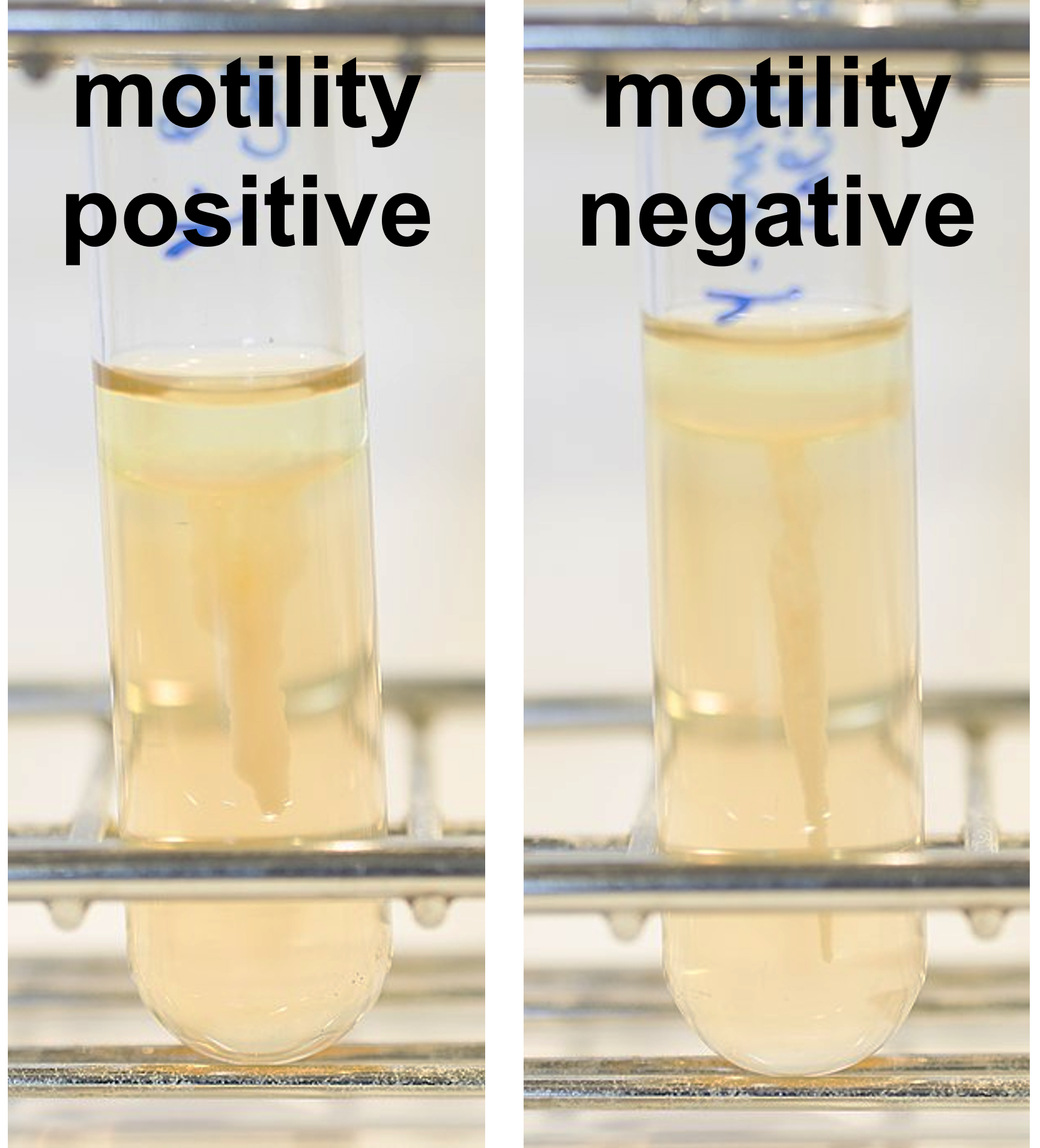
Figure 7: SIM deep results shown with Kovac's reagent present on top of the medium. (Left) Bacterial growth has moved away from the stab and therefore is motility positive. The Kovac's reagent is not red and therefore is indole negative. The medium did not produce any black coloration and is therefore hydrogen sulfide reduction negative. (Right) Bacterial growth stayed close to the initial stab and there is is motility negative. The Kovac's reagent is not red and therefore is indole negative. The medium did not produce any black coloration and is therefore hydrogen sulfide reduction negative.
Laboratory Instructions
- Obtain a deep of SIM medium.
- Label the test tube with your group name, the medium name, and the bacterial species name.
- Using an inoculating needle, stab the medium about 2/3 of the way down and out the same pathway as quickly as possible with the bacterial species provided by your instructor.
- Repeat steps 1-3 if testing multiple bacterial species.
- Incubate the tube for at least 48 hours.
- After the incubation period, examine your tube.
Indole Test
- After the SIM deep has incubated for at least 48 hours, examine results for motility and hydrogen sulfide production and record results.
- Add 10 drops of Kovac’s reagent to the top of the SIM meidum tube.
- if Kovac's reagent produces a dark yellow ring indicates the species is indole negative
- if Kovac's reagent produces a red ring indicates the species is indole positive
Results & Questions
| bacterial species | medium color | hydrogen sulfide production (+/-) | Kovac's reagent color | indole (+/-) | location of growth (along stab / fanned out) | motility (+/-) |
|---|---|---|---|---|---|---|
| Escherichia coli | ||||||
| Proteus vulgaris | ||||||
| Staphylococcus aureus |
- Complete the table above with results from the SIM deep tests.
- A bacterial species that is positive for hydrogen sulfide production could be producing H2S in one of two ways. What are these?
- Is hydrogen sulfide black? Explain your answer.
- A bacterial species that is positive for indole produces what enzyme? What does this enzyme do?
- What is the role of Kovac's reagent in the indole test?
- A bacterial species that is positive motility has what type of bacterial cell structure found only in some cells?
- Is using a SIM deep useful for bacterial species identification and characterization? Explain your answer.
Attributions
- General Microbiology Lab Manual (Pakpour & Horgan) by Nazzy Pakpour & Sharon Horgan is licensed under CC BY-SA 4.0
- Klamm’s Microbiology Laboratory Manual by Loretta Sanderson Klamm is licensed under CC BY-NC-SA 4.0
- Laboratory Exercises in Microbiology: Discovering the Unseen World Through Hands-On Investigation by Susan McLaughlin and Joan Petersen is licensed under CC BY-NC
- Microbiology for Allied Health Students: Lab Manual by Molly Smith and Sara Selby (GALILEO Open Learning Materials) is licensed under CC BY 4.0
- Red Mountain Microbiology by Jill Raymond Ph.D.; Graham Boorse, Ph.D.; Anne Mason M.S. is licensed under CC BY-NC 4.0
- Yersinia enterocolitica in SIM Agar1 125.jpg by A doubt is licensed under CC BY-SA 4.0
- Yersinia enterocolitica in SIM Agar2 123.jpg by A doubt is licensed under CC BY-SA 4.0


