14: Chemolithotrophy & Nitrogen Metabolism
- Page ID
- 10702
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Chemolithotrophy
Chemolithotrophy is the oxidation of inorganic chemicals for the generation of energy. The process can use oxidative phosphorylation, just like aerobic and anaerobic respiration, but now the substance being oxidized (the electron donor) is an inorganic compound. The electrons are passed off to carriers within the electron transport chain, generating a proton motive force that is used to generate ATP with the help of ATP synthase.
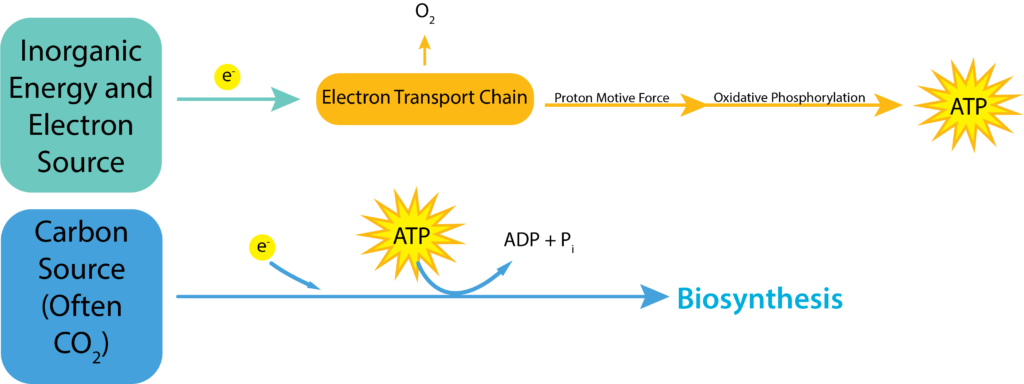
Chemolithotrophy Pathways.
Electrons donors
Chemolithotrophs use a variety of inorganic compounds as electron donors, with the most common substances being hydrogen gas, sulfur compounds (such as sulfide and sulfur), nitrogen compounds (such as ammonium and nitrite), and ferrous iron.
- Hydrogen oxidizers – these organisms oxidize hydrogen gas (H2) with the use of a hydrogenase enzyme. Both aerobic and anaerobic hydrogen oxidizers exist, with the aerobic organisms eventually reducing oxygen to water.
- Sulfur oxidizers – as a group these organisms are capable of oxidizing a wide variety of reduced and partially reduced sulfur compounds such as hydrogen sulfide (H2S), elemental sulfur (S0), thiosulfate (S2O32-), and sulfite (SO32-). Sulfate (SO42-) is frequently a by-product of the oxidation. Often the oxidation occurs in a stepwise fashion with the help of the sulfite oxidase enzyme.
- Nitrogen oxidizers – the oxidation of ammonia (NH3) is performed as a two-step process by nitrifying microbes, where one group oxidizes ammonia to nitrite (NO2-) and the second group oxidizes the nitrite to nitrate (NO3-). The entire process is known as nitrification and is performed by small groups of aerobic bacteria and archaea, often found living together in soil or in water systems.
- Iron oxidizers – these organisms oxidize ferrous iron (Fe2+) to ferric iron (Fe3+). Since Fe2+ has such a positive standard reduction potential, the bioenergetics are not extremely favorable, even using oxygen as a final electron acceptor. The situation is made more difficult for these organisms by the fact that Fe2+ spontaneously oxidizes to Fe3+ in the presence of oxygen; the organisms must use it for their own purposes before that happens.
Electron acceptors
Chemolithotrophy can occur aerobically or anaerobically. Just as with either type of respiration, the best electron acceptor is oxygen, to create the biggest distance between the electron donor and the electron acceptor. Using a non-oxygen acceptor allows chemolithotrophs to have greater diversity and the ability to live in a wider variety of environments, although they sacrifice energy production.
Amount of ATP generated
Just as both the electron donors and acceptors can vary widely for this group of organisms, the amount of ATP generated for their efforts will vary widely as well. They will not make as much ATP as an organism using aerobic respiration, since the largest ΔE0’ is found using glucose as an electron donor and oxygen as an electron acceptor. But how much less than 32 molecules of ATP greatly depends upon the actual donor and acceptor being used. The smaller the distance between the two, the less ATP that will be formed.
Chemolithoautotrophs vs chemolithoheterotrophs
Most chemolithotrophs are autotrophs (chemolithoautotrophs), where they fix atmospheric carbon dioxide to assemble the organic compounds that they need. These organisms require both ATP and reducing power (i.e. NADH/NADPH) in order to ultimately convert the oxidized molecule CO2 into a greatly reduced organic compound, like glucose. If a chemolithoautotroph is using an electron donor with a higher redox potential than NAD+/NADP, they must use reverse electron flow to push electrons back up the electron tower. This is energetically unfavorable to the cell, consuming energy from the proton motive force to drive electrons in a reverse direction back through the ETC.
Some microbes are chemolithoheterotrophs, using an inorganic chemical for their energy and electron needs, but relying on organic chemicals in the environment for their carbon needs. These organisms are also called mixotrophs, since they require both inorganic and chemical compounds for their growth and reproduction.
Nitrogen Metabolism
The nitrogen cycle depicts the different ways in which nitrogen, an essential element for life, is used and converted by organisms for various purposes. Much of the chemical conversions are performed by microbes as part of their metabolism, performing a valuable service in the process for other organisms in providing them with an alternate chemical form of the element.
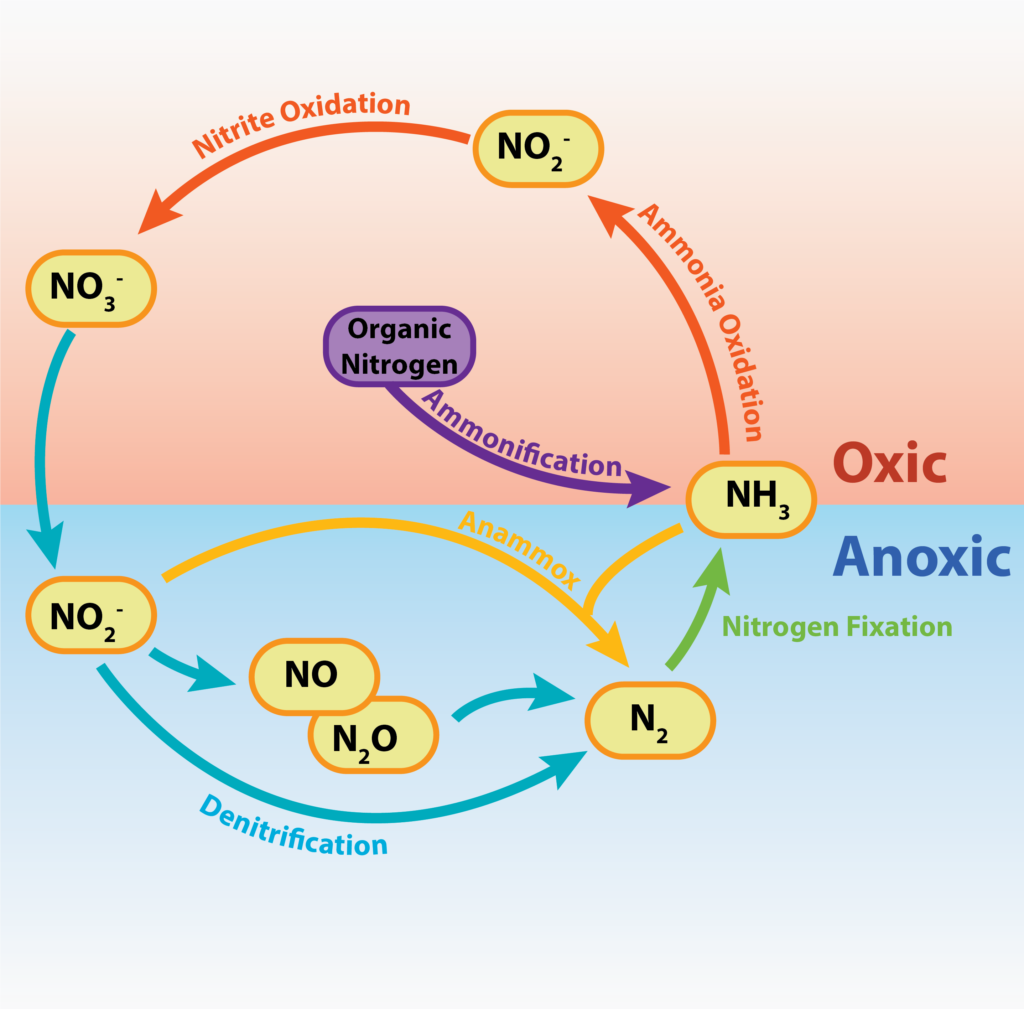
Nitrogen Cycle.
Nitrogen Fixation
Nitrogen fixation describes the conversion of the relatively inert dinitrogen gas (N2) into ammonia (NH3), a much more useable form of nitrogen for most life forms. The process is performed by diazotrophs, a limited number of bacteria and archaea that can grow without an external source of fixed nitrogen, because of their abilities. Nitrogen fixation is an essential process for Earth’s organisms, since nitrogen is a required component of various organic molecules, such as amino acids and nucleotides. Plants, animals, and other organisms rely on bacteria and archaea to provide nitrogen in a fixed form, since no eukaryote is known that can fix nitrogen.
Nitrogen fixation is an extremely energy and electron intensive process, in order to break the triple bond in N2 and reduce it to NH3. It requires a particular enzyme known as nitrogenase, which is inactivated by O2. Thus, nitrogen fixation must take place in an anaerobic environment. Aerobic nitrogen-fixing organisms must devise special conditions or arrangements in order to protect their enzyme. Nitrogen-fixing organisms can either exist independently or pair up with a plant host:
- Symbiotic nitrogen-fixing organisms: these bacteria partner up with a plant, to provide them with an environment appropriate for the functioning of their nitrogenase enzyme. The bacteria live in the plant’s tissue, often in root nodules, fixing nitrogen and sharing the results. The plant provides both the location to fix nitrogen, as well as additional nutrients to support the energy-taxing process of nitrogen fixation. It has been shown that the bacteria and the host exchange chemical recognition signals that facilitate the relationship. One of the best known bacteria in this category is Rhizobium, which partners up with plants of the legume family (clover, soybeans, alfalfa, etc).
- Free-living nitrogen-fixing organisms: these organisms, both bacteria and archaea, fix nitrogen for their own use that ends up being shared when the organisms dies or is ingested. Free-living nitrogen-fixing organisms that grow anaerobically do not have to worry about special adaptations for their nitrogenase enzyme. Aerobic organisms must make adaptations. Cyanobacteria, a multicellular bacterium, make specialized cells known as heterocystsin which nitrogen fixation occurs. Since Cyanobacteria produce oxygen as part of their photosynthesis, an anoxygenic version occurs within the heterocyst, allowing the nitrogenase to remain active. The heterocysts share the fixed nitrogen with surrounding cells, while the surrounding cells provide additional nutrients to the heterocysts.
Assimilation
Assimilation is a reductive process by which an inorganic form of nitrogen is reduced to organic nitrogen compounds such as amino acids and nucleotides, allowing for cellular growth and reproduction. Only the amount needed by the cell is reduced. Ammonia assimilation occurs when the ammonia (NH3)/ammonium ion (NH4+) formed during nitrogen fixation is incorporated into cellular nitrogen. Assimilative nitrate reduction is a reduction of nitrate to cellular nitrogen, in a multi-step process where nitrate is reduced to nitrite then ammonia and finally into organic nitrogen.
Nitrification
As mentioned above, nitrification is performed by chemolithotrophs using a reduced or partially reduced form of nitrogen as an electron donor to obtain energy. ATP is gained by the process of oxidative phosphorylation, using a ETC, PMF, and ATP synthase.
Denitrification
Denitrification refers to the reduction of NO3- to gaseous nitrogen compounds, such as N2. Denitrifying microbes perform anaerobic respiration, using NO3- as an alternate final electron acceptor to O2. This is a type of dissimilatory nitrate reduction where the nitrate is being reduced during energy conservation, not for the purposes of making organic compounds. This produces large amounts of excess byproducts, resulting in the loss of nitrogen from the local environment to the atmosphere.
Anammox
Anammox or anaerobic ammonia oxidation is performed by marine bacteria, relatively recently discovered, that utilize nitrogen compounds as both electron acceptor and electron donor. Ammonia is oxidized anaerobically as the electron donor while nitrite is utilized as the electron acceptor, with dinitrogen gas produced as a byproduct. The reactions occur within the anammoxosome, a specialized cytoplasmic structure which constitutes 50-70% of the total cell volume. Just like denitrification, the anammox reaction removes fixed nitrogen from a local environment, releasing it to the atmosphere.
Key Words
chemolithotrophy, hydrogen oxidizers, hydrogenase, sulfur oxidizers, sulfite oxidase, nitrogen oxidizers, nitrification, iron oxidizers, chemolithoautotroph, reverse electron flow, chemolithoheterotroph, mixotroph, nitrogen fixation, diazotroph, nitrogenase, symbiotic nitrogen-fixing organisms, Rhizobium, legume, free-living nitrogen-fixing organisms, Cyanobacteria, heterocyst, assimilation, ammonia assimilation, assimilative nitrate reduction, denitrification, dissimilatory nitrate reduction, anammox, anaerobic ammonia oxidation, anammoxosome.
Study Questions
- What is chemolithotrophy?
- What are the most common electron donors and acceptors for chemolithotrophs? How does their amount of ATP produced compare to chemoorganotrophs?
- How do chemolithoautotrophs and chemolithoheterotrophs differ? What is the reverse electron flow and how/why is it used by some chemolithoautotrophs?
- What roles do bacteria/archaea play in the nitrogen cycle? How are different nitrogen compounds used in their metabolism?
- What is required for nitrogen fixation? How do free living nitrogen fixers and plant associated nitrogen fixers differ? How do Rhizobium and Cyanobacteria protect their nitrogenase from oxygen?
- What are the different mechanisms of nitrogen metabolism? What conversion is occurring for each? What is the purpose of each and how does it relate to the organism’s metabolism?
Nitrogen Cycle.
Nitrogen Fixation
Nitrogen fixation describes the conversion of the relatively inert dinitrogen gas (N2) into ammonia (NH3), a much more useable form of nitrogen for most life forms. The process is performed by diazotrophs, a limited number of bacteria and archaea that can grow without an external source of fixed nitrogen, because of their abilities. Nitrogen fixation is an essential process for Earth’s organisms, since nitrogen is a required component of various organic molecules, such as amino acids and nucleotides. Plants, animals, and other organisms rely on bacteria and archaea to provide nitrogen in a fixed form, since no eukaryote is known that can fix nitrogen.
Nitrogen fixation is an extremely energy and electron intensive process, in order to break the triple bond in N2 and reduce it to NH3. It requires a particular enzyme known as nitrogenase, which is inactivated by O2. Thus, nitrogen fixation must take place in an anaerobic environment. Aerobic nitrogen-fixing organisms must devise special conditions or arrangements in order to protect their enzyme. Nitrogen-fixing organisms can either exist independently or pair up with a plant host:
- Symbiotic nitrogen-fixing organisms: these bacteria partner up with a plant, to provide them with an environment appropriate for the functioning of their nitrogenase enzyme. The bacteria live in the plant’s tissue, often in root nodules, fixing nitrogen and sharing the results. The plant provides both the location to fix nitrogen, as well as additional nutrients to support the energy-taxing process of nitrogen fixation. It has been shown that the bacteria and the host exchange chemical recognition signals that facilitate the relationship. One of the best known bacteria in this category is Rhizobium, which partners up with plants of the legume family (clover, soybeans, alfalfa, etc).
- Free-living nitrogen-fixing organisms: these organisms, both bacteria and archaea, fix nitrogen for their own use that ends up being shared when the organisms dies or is ingested. Free-living nitrogen-fixing organisms that grow anaerobically do not have to worry about special adaptations for their nitrogenase enzyme. Aerobic organisms must make adaptations. Cyanobacteria, a multicellular bacterium, make specialized cells known as heterocystsin which nitrogen fixation occurs. Since Cyanobacteria produce oxygen as part of their photosynthesis, an anoxygenic version occurs within the heterocyst, allowing the nitrogenase to remain active. The heterocysts share the fixed nitrogen with surrounding cells, while the surrounding cells provide additional nutrients to the heterocysts.
Assimilation
Assimilation is a reductive process by which an inorganic form of nitrogen is reduced to organic nitrogen compounds such as amino acids and nucleotides, allowing for cellular growth and reproduction. Only the amount needed by the cell is reduced. Ammonia assimilation occurs when the ammonia (NH3)/ammonium ion (NH4+) formed during nitrogen fixation is incorporated into cellular nitrogen. Assimilative nitrate reduction is a reduction of nitrate to cellular nitrogen, in a multi-step process where nitrate is reduced to nitrite then ammonia and finally into organic nitrogen.
Nitrification
As mentioned above, nitrification is performed by chemolithotrophs using a reduced or partially reduced form of nitrogen as an electron donor to obtain energy. ATP is gained by the process of oxidative phosphorylation, using a ETC, PMF, and ATP synthase.
Denitrification
Denitrification refers to the reduction of NO3- to gaseous nitrogen compounds, such as N2. Denitrifying microbes perform anaerobic respiration, using NO3- as an alternate final electron acceptor to O2. This is a type of dissimilatory nitrate reduction where the nitrate is being reduced during energy conservation, not for the purposes of making organic compounds. This produces large amounts of excess byproducts, resulting in the loss of nitrogen from the local environment to the atmosphere.
Anammox
Anammox or anaerobic ammonia oxidation is performed by marine bacteria, relatively recently discovered, that utilize nitrogen compounds as both electron acceptor and electron donor. Ammonia is oxidized anaerobically as the electron donor while nitrite is utilized as the electron acceptor, with dinitrogen gas produced as a byproduct. The reactions occur within the anammoxosome, a specialized cytoplasmic structure which constitutes 50-70% of the total cell volume. Just like denitrification, the anammox reaction removes fixed nitrogen from a local environment, releasing it to the atmosphere.
Key Words
chemolithotrophy, hydrogen oxidizers, hydrogenase, sulfur oxidizers, sulfite oxidase, nitrogen oxidizers, nitrification, iron oxidizers, chemolithoautotroph, reverse electron flow, chemolithoheterotroph, mixotroph, nitrogen fixation, diazotroph, nitrogenase, symbiotic nitrogen-fixing organisms, Rhizobium, legume, free-living nitrogen-fixing organisms, Cyanobacteria, heterocyst, assimilation, ammonia assimilation, assimilative nitrate reduction, denitrification, dissimilatory nitrate reduction, anammox, anaerobic ammonia oxidation, anammoxosome.
Study Questions
- What is chemolithotrophy?
- What are the most common electron donors and acceptors for chemolithotrophs? How does their amount of ATP produced compare to chemoorganotrophs?
- How do chemolithoautotrophs and chemolithoheterotrophs differ? What is the reverse electron flow and how/why is it used by some chemolithoautotrophs?
- What roles do bacteria/archaea play in the nitrogen cycle? How are different nitrogen compounds used in their metabolism?
- What is required for nitrogen fixation? How do free living nitrogen fixers and plant associated nitrogen fixers differ? How do Rhizobium and Cyanobacteria protect their nitrogenase from oxygen?
- What are the different mechanisms of nitrogen metabolism? What conversion is occurring for each? What is the purpose of each and how does it relate to the organism’s metabolism?


