20.3: Agglutination Assays
- Page ID
- 5239
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Compare direct and indirect agglutination
- Identify various uses of hemagglutination in the diagnosis of disease
- Explain how blood types are determined
- Explain the steps used to cross-match blood to be used in a transfusion
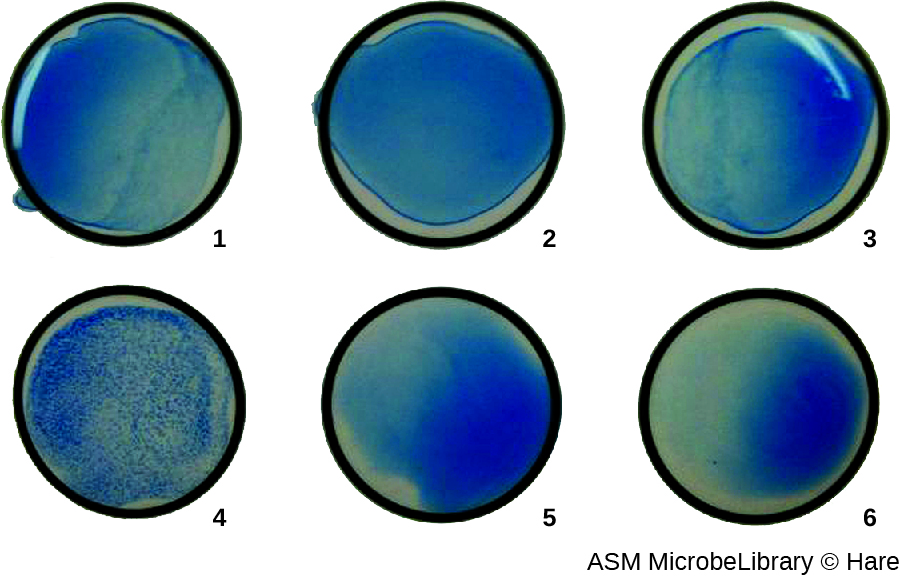
In addition to causing precipitation of soluble molecules and flocculation of molecules in suspension, antibodies can also clump together cells or particles (e.g., antigen-coated latex beads) in a process called agglutination (Figure 18.1.8). Agglutination can be used as an indicator of the presence of antibodies against bacteria or red blood cells. Agglutination assays are usually quick and easy to perform on a glass slide or microtiter plate (Figure \(\PageIndex{1}\)). Microtiter plates have an array of wells to hold small volumes of reagents and to observe reactions (e.g., agglutination) either visually or using a specially designed spectrophotometer. The wells come in many different sizes for assays involving different volumes of reagents.

Agglutination of Bacteria and Viruses
The use of agglutination tests to identify streptococcal bacteria was developed in the 1920s by Rebecca Lancefieldworking with her colleagues A.R. Dochez and Oswald Avery.1 She used antibodies to identify M protein, a virulence factor on streptococci that is necessary for the bacteria’s ability to cause strep throat. Production of antibodies against M protein is crucial in mounting a protective response against the bacteria.
Lancefield used antisera to show that different strains of the same species of streptococci express different versions of M protein, which explains why children can come down with strep throat repeatedly. Lancefield classified beta-hemolytic streptococci into many groups based on antigenic differences in group-specific polysaccharides located in the bacterial cell wall. The strains are called serovars because they are differentiated using antisera. Identifying the serovars present in a disease outbreak is important because some serovars may cause more severe disease than others.
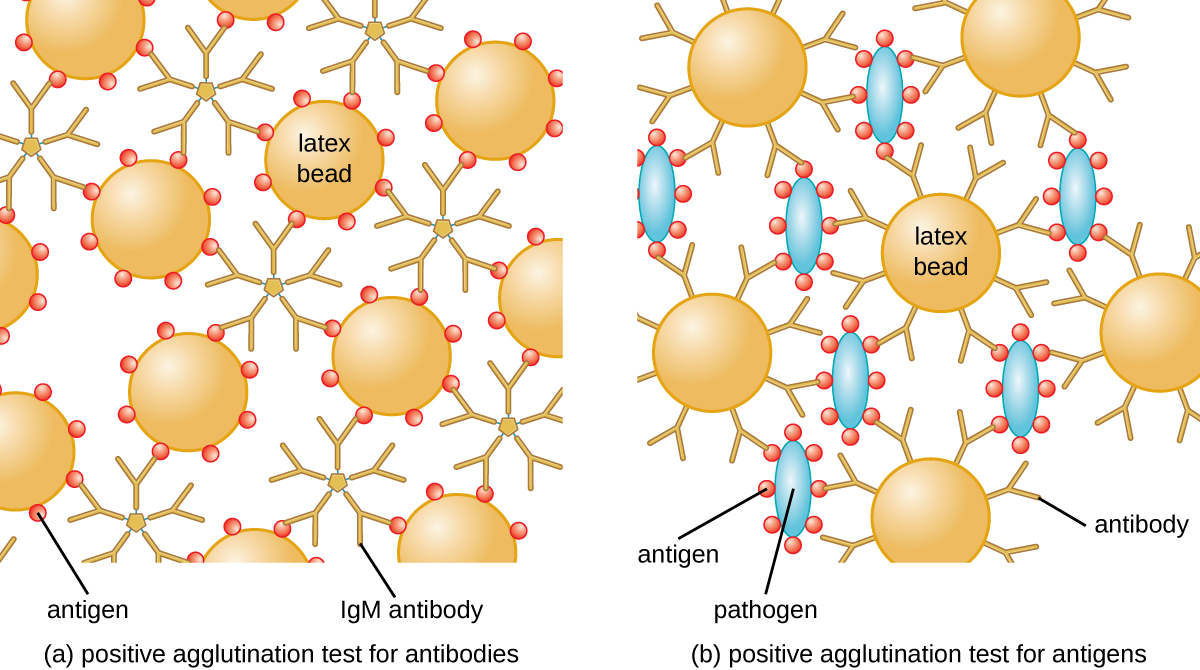
The method developed by Lancefield is a direct agglutination assay, since the bacterial cells themselves agglutinate. A similar strategy is more commonly used today when identifying serovars of bacteria and viruses; however, to improve visualization of the agglutination, the antibodies may be attached to inert latex beads. This technique is called an indirect agglutination assay (or latex fixation assay), because the agglutination of the beads is a marker for antibody binding to some other antigen (Figure \(\PageIndex{2}\)). Indirect assays can be used to detect the presence of either antibodies or specific antigens.
To identify antibodies in a patient’s serum, the antigen of interest is attached to latex beads. When mixed with patient serum, the antibodies will bind the antigen, cross-linking the latex beads and causing the beads to agglutinate indirectly; this indicates the presence of the antibody (Figure \(\PageIndex{3}\)). This technique is most often used when looking for IgM antibodies, because their structure provides maximum cross-linking. One widely used example of this assay is a test for rheumatoid factor (RF) to confirm a diagnosis of rheumatoid arthritis. RF is, in fact, the presence of IgM antibodies that bind to the patient’s own IgG. RF will agglutinate IgG-coated latex beads.
In the reverse test, soluble antigens can be detected in a patient’s serum by attaching specific antibodies (commonly mAbs) to the latex beads and mixing this complex with the serum (Figure \(\PageIndex{3}\)).
Agglutination tests are widely used in underdeveloped countries that may lack appropriate facilities for culturing bacteria. For example, the Widal test, used for the diagnosis of typhoid fever, looks for agglutination of Salmonella enterica subspecies typhi in patient sera. The Widal test is rapid, inexpensive, and useful for monitoring the extent of an outbreak; however, it is not as accurate as tests that involve culturing of the bacteria. The Widal test frequently produces false positives in patients with previous infections with other subspecies of Salmonella, as well as false negatives in patients with hyperproteinemia or immune deficiencies.
In addition, agglutination tests are limited by the fact that patients generally do not produce detectable levels of antibody during the first week (or longer) of an infection. A patient is said to have undergone seroconversion when antibody levels reach the threshold for detection. Typically, seroconversion coincides with the onset of signs and symptoms of disease. However, in an HIV infection, for example, it generally takes 3 weeks for seroconversion to take place, and in some instances, it may take much longer.
Similar to techniques for the precipitin ring test and plaque assays, it is routine to prepare serial two-fold dilutions of the patient’s serum and determine the titer of agglutinating antibody present. Since antibody levels change over time in both primary and secondary immune responses, by checking samples over time, changes in antibody titer can be detected. For example, a comparison of the titer during the acute phase of an infection versus the titer from the convalescent phase will distinguish whether an infection is current or has occurred in the past. It is also possible to monitor how well the patient’s immune system is responding to the pathogen.
Watch this video that demonstrates agglutination reactions with latex beads.
- How is agglutination used to distinguish serovars from each other?
- In a latex bead assay to test for antibodies in a patient's serum, with what are the beads coated?
- What has happened when a patient has undergone seroconversion?
Hemagglutination
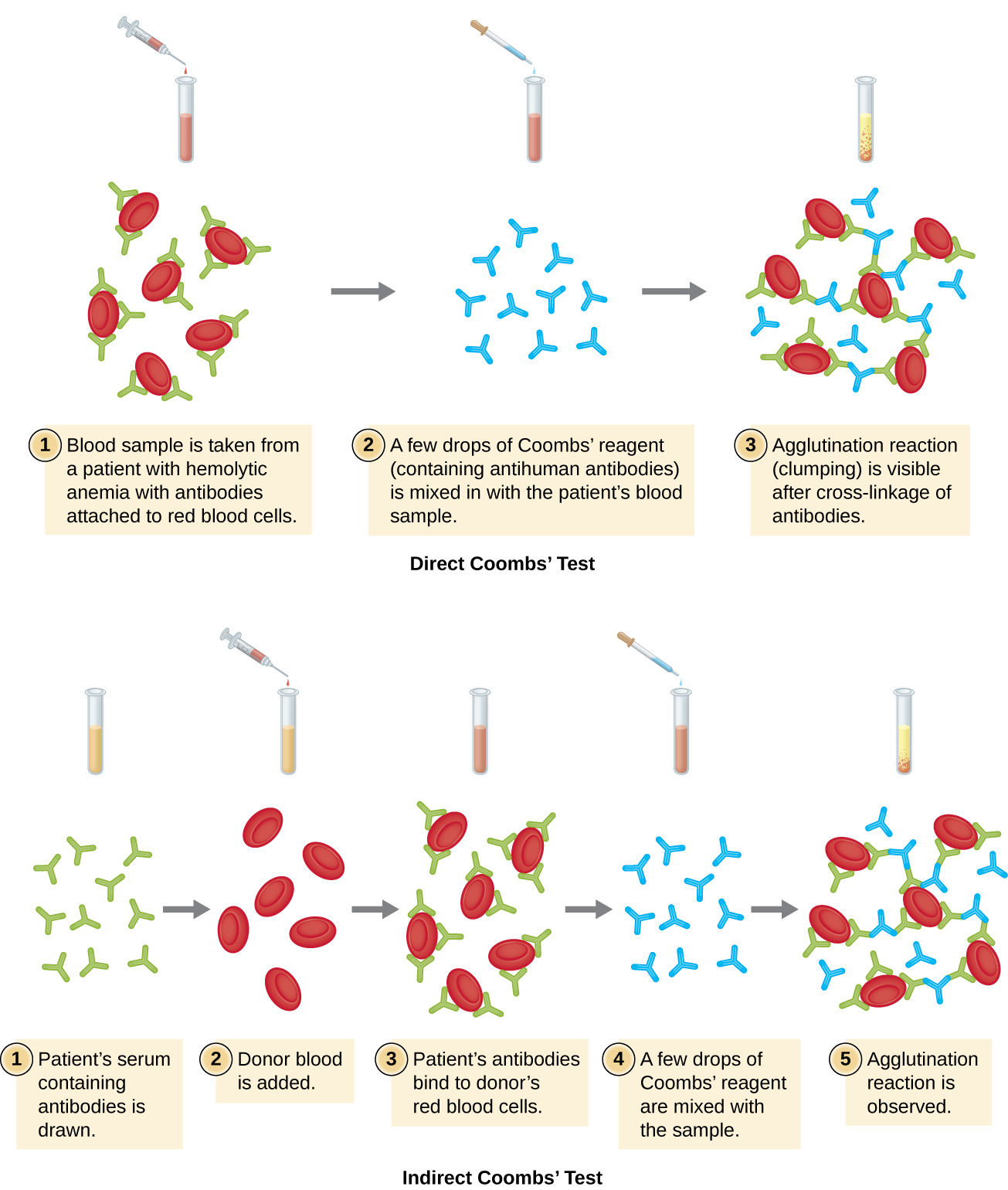
Agglutination of red blood cells is called hemagglutination. One common assay that uses hemagglutination is the direct Coombs’ test, also called the direct antihuman globulin test (DAT), which generally looks for nonagglutinating antibodies. The test can also detect complement attached to red blood cells.
The Coombs’ test is often employed when a newborn has jaundice, yellowing of the skin caused by high blood concentrations of bilirubin, a product of the breakdown of hemoglobin in the blood. The Coombs’ test is used to determine whether the child’s red blood cells have been bound by the mother’s antibodies. These antibodies would activate complement, leading to red blood cell lysis and the subsequent jaundice. Other conditions that can cause positive direct Coombs’ tests include hemolytic transfusion reactions, autoimmune hemolytic anemia, infectious mononucleosis (caused by Epstein-Barr virus), syphilis, and Mycoplasma pneumonia. A positive direct Coombs’ test may also be seen in some cancers and as an allergic reaction to some drugs (e.g., penicillin).
The antibodies bound to red blood cells in these conditions are most often IgG, and because of the orientation of the antigen-binding sites on IgG and the comparatively large size of a red blood cell, it is unlikely that any visible agglutination will occur. However, the presence of IgG bound to red blood cells can be detected by adding Coombs’ reagent, an antiserum containing antihuman IgG antibodies (that may be combined with anti-complement) (Figure \(\PageIndex{4}\)). The Coombs’ reagent links the IgG attached to neighboring red blood cells and thus promotes agglutination.
There is also an indirect Coombs’ test known as the indirect antiglobulin test (IAT). This screens an individual for antibodies against red blood cell antigens (other than the A and B antigens) that are unbound in a patient’s serum (Figure \(\PageIndex{4}\)). IAT can be used to screen pregnant women for antibodies that may cause hemolytic disease of the newborn. It can also be used prior to giving blood transfusions. More detail on how the IAT is performed is discussed below.
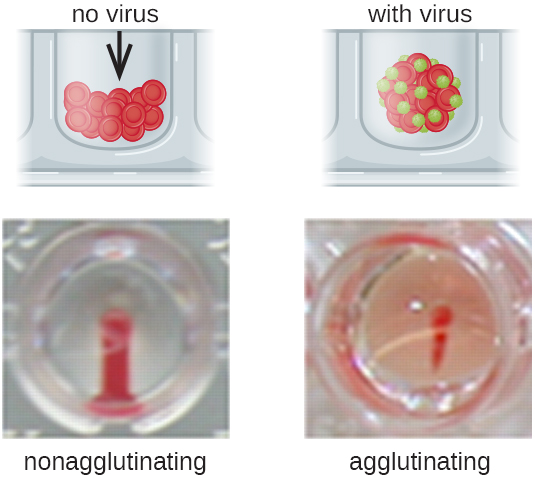
Antibodies that bind to red blood cells are not the only cause of hemagglutination. Some viruses also bind to red blood cells, and this binding can cause agglutination when the viruses cross-link the red blood cells. For example, influenza viruses have two different types of viral spikes called neuraminidase (N) and hemagglutinin (H), the latter named for its ability to agglutinate red blood cells (see Viruses). Thus, we can use red blood cells to detect the presence of influenza virus by direct hemagglutination assays (HA), in which the virus causes visible agglutination of red blood cells. The mumps and rubella viruses can also be detected using HA.
Most frequently, a serial dilution viral agglutination assay is used to measure the titer or estimate the amount of virus produced in cell culture or for vaccine production. A viral titer can be determined using a direct HA by making a serial dilution of the sample containing the virus, starting with a high concentration of sample that is then diluted in a series of wells. The highest dilution producing visible agglutination is the titer. The assay is carried out in a microtiter plate with V- or round-bottomed wells. In the presence of agglutinating viruses, the red blood cells and virus clump together and produce a diffuse mat over the bottom of the well. In the absence of virus, the red blood cells roll or sediment to the bottom of the well and form a dense pellet, which is why flat-bottomed wells cannot be used (Figure \(\PageIndex{5}\)).
A modification of the HA assay can be used to determine the titer of antiviral antibodies. The presence of these antibodies in a patient’s serum or in a lab-produced antiserum will neutralize the virus and block it from agglutinating the red cells, making this a viral hemagglutination inhibition assay (HIA). In this assay, patient serum is mixed with a standardized amount of virus. After a short incubation, a standardized amount of red blood cells is added and hemagglutination is observed. The titer of the patient’s serum is the highest dilution that blocks agglutination (Figure \(\PageIndex{6}\)).
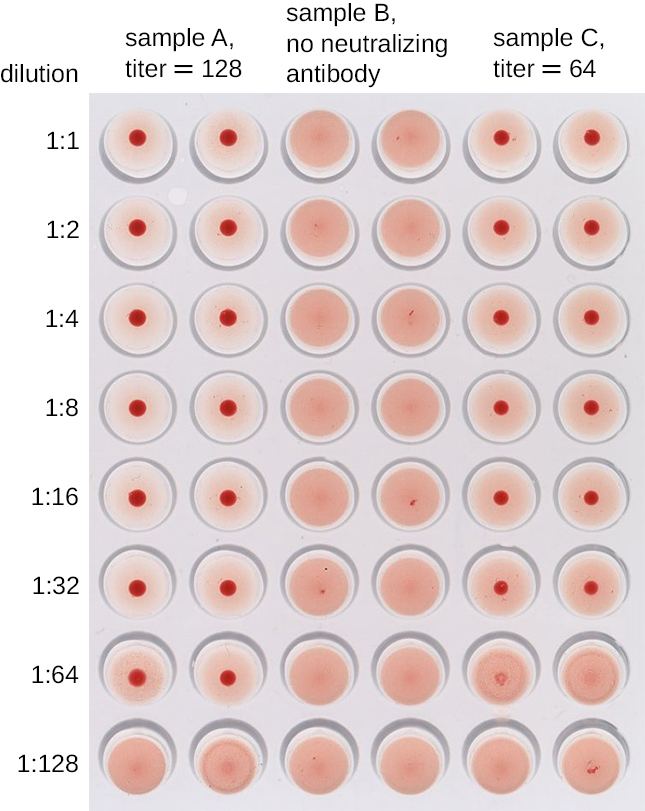
- What is the mechanism by which viruses are detected in a hemagglutination assay?
- Which hemagglutination result tells us the titer of virus in a sample?
Much of what we know today about the human immune system has been learned through research conducted using animals—primarily, mammals—as models. Besides research, mammals are also used for the production of most of the antibodies and other immune system components needed for immunodiagnostics. Vaccines, diagnostics, therapies, and translational medicine in general have all been developed through research with animal models.
Consider some of the common uses of laboratory animals for producing immune system components. Guinea pigs are used as a source of complement, and mice are the primary source of cells for making mAbs. These mAbs can be used in research and for therapeutic purposes. Antisera are raised in a variety of species, including horses, sheep, goats, and rabbits. When producing an antiserum, the animal will usually be injected at least twice, and adjuvants may be used to boost the antibody response. The larger animals used for making antisera will have blood harvested repeatedly over long periods of time, with little harm to the animals, but that is not usually the case for rabbits. Although we can obtain a few milliliters of blood from the ear veins of rabbits, we usually need larger volumes, which results in the deaths of the animals.
We also use animals for the study of disease. The only way to grow Treponema pallidum for the study of syphilis is in living animals. Many viruses can be grown in cell culture, but growth in cell culture tells us very little about how the immune system will respond to the virus. When working on a newly discovered disease, we still employ Koch’s postulates, which require causing disease in lab animals using pathogens from pure culture as a crucial step in proving that a particular microorganism is the cause of a disease. Studying the proliferation of bacteria and viruses in animal hosts, and how the host immune system responds, has been central to microbiological research for well over 100 years.
While the practice of using laboratory animals is essential to scientific research and medical diagnostics, many people strongly object to the exploitation of animals for human benefit. This ethical argument is not a new one—indeed, one of Charles Darwin's daughters was an active antivivisectionist (vivisection is the practice of cutting or dissecting a live animal to study it). Most scientists acknowledge that there should be limits on the extent to which animals can be exploited for research purposes. Ethical considerations have led the National Institutes of Health (NIH) to develop strict regulations on the types of research that may be performed. These regulations also include guidelines for the humane treatment of lab animals, setting standards for their housing, care, and euthanization. The NIH document “Guide for the Care and Use of Laboratory Animals” makes it clear that the use of animals in research is a privilege granted by society to researchers.
The NIH guidelines are based on the principle of the three R’s: replace, refine, and reduce. Researchers should strive to replace animal models with nonliving models, replace vertebrates with invertebrates whenever possible, or use computer-models when applicable. They should refine husbandry and experimental procedures to reduce pain and suffering, and use experimental designs and procedures that reduce the number of animals needed to obtain the desired information. To obtain funding, researchers must satisfy NIH reviewers that the research justifies the use of animals and that their use is in accordance with the guidelines.
At the local level, any facility that uses animals and receives federal funding must have an Institutional Animal Care and Use Committee (IACUC) that ensures that the NIH guidelines are being followed. The IACUC must include researchers, administrators, a veterinarian, and at least one person with no ties to the institution, that is, a concerned citizen. This committee also performs inspections of laboratories and protocols. For research involving human subjects, an Institutional Review Board (IRB) ensures that proper guidelines are followed.
Visit this site to view the NIH Guide for the Care and Use of Laboratory Animals.
Blood Typing and Cross-Matching
In addition to antibodies against bacteria and viruses to which they have previously been exposed, most individuals also carry antibodies against blood types other than their own. There are presently 33 immunologically important blood-type systems, many of which are restricted within various ethnic groups or rarely result in the production of antibodies. The most important and perhaps best known are the ABO and Rh blood groups (see Figure 19.1.3).
When units of blood are being considered for transfusion, pretransfusion blood testing must be performed. For the blood unit, commercially prepared antibodies against the A, B, and Rh antigens are mixed with red blood cells from the units to initially confirm that the blood type on the unit is accurate. Once a unit of blood has been requested for transfusion, it is vitally important to make sure the donor (unit of blood) and recipient (patient) are compatible for these crucial antigens. In addition to confirming the blood type of the unit, the patient’s blood type is also confirmed using the same commercially prepared antibodies to A, B, and Rh. For example, as shown in Figure \(\PageIndex{7}\), if the donor blood is A-positive, it will agglutinate with the anti-A antiserum and with the anti-Rh antiserum. If no agglutination is observed with any of the sera, then the blood type would be O-negative.
Following determination of the blood type, immediately prior to releasing the blood for transfusion, a cross-match is performed in which a small aliquot of the donor red blood cells are mixed with serum from the patient awaiting transfusion. If the patient does have antibodies against the donor red blood cells, hemagglutination will occur. To confirm any negative test results and check for sensitized red blood cells, Coombs’ reagent may be added to the mix to facilitate visualization of the antibody-red blood cell interaction.
Under some circumstances, a minor cross-match may be performed as well. In this assay, a small aliquot of donor serum is mixed with patient red blood cells. This allows the detection of agglutinizing antibodies in the donor serum. This test is rarely necessary because transfusions generally use packed red blood cells with most of the plasma removed by centrifugation.
Red blood cells have many other antigens in addition to ABO and Rh. While most people are unlikely to have antibodies against these antigens, women who have had multiple pregnancies or patients who have had multiple transfusions may have them because of repeated exposure. For this reason, an antibody screen test is used to determine if such antibodies are present. Patient serum is checked against commercially prepared, pooled, type O red blood cells that express these antigens. If agglutination occurs, the antigen to which the patient is responding must be identified and determined not to be present in the donor unit.

- If a patient's blood agglutinates with anti-B serum, what is the patient’s blood type?
- What is a cross-match assay, and why is it performed?
Table \(\PageIndex{1}\) summarizes the various kinds of agglutination assays discussed in this section.
| Type of Assay | Mechanism | Example |
|---|---|---|
| Agglutination | Direct: Antibody is used to clump bacterial cells or other large structures | Serotyping bacteria |
| Indirect: Latex beads are coupled with antigen or antibody to look for antibody or antigen, respectively, in patient serum | Confirming the presence of rheumatoid factor (IgM-binding Ig) in patient serum | |
| Hemagglutination | Direct: Some bacteria and viruses cross-link red blood cells and clump them together | Diagnosing influenza, mumps, and measles |
| Direct Coombs’ test (DAT): Detects nonagglutinating antibodies or complement proteins on red blood cells in vivo | Checking for maternal antibodies binding to neonatal red blood cells | |
| Indirect Coombs’ test (IAT): Screens an individual for antibodies against red blood cell antigens (other than the A and B antigens) that are unbound in a patient’s serum in vitro | Performing pretransfusion blood testing | |
| Viral hemagglutination inhibition: Uses antibodies from a patient to inhibit viral agglutination | Diagnosing various viral diseases by the presence of patient antibodies against the virus | |
| Blood typing and cross-matching: Detects ABO, Rh, and minor antigens in the blood | Matches donor blood to recipient immune requirements |
Key Concepts and Summary
- Antibodies can agglutinate cells or large particles into a visible matrix. Agglutination tests are often done on cards or in microtiter plates that allow multiple reactions to take place side by side using small volumes of reagents.
- Using antisera against certain proteins allows identification of serovars within species of bacteria.
- Detecting antibodies against a pathogen can be a powerful tool for diagnosing disease, but there is a period of time before patients go through seroconversion and the level of antibodies becomes detectable.
- Agglutination of latex beads in indirect agglutination assays can be used to detect the presence of specific antigens or specific antibodies in patient serum.
- The presence of some antibacterial and antiviral antibodies can be confirmed by the use of the direct Coombs’ test, which uses Coombs’ reagent to cross-link antibodies bound to red blood cells and facilitate hemagglutination.
- Some viruses and bacteria will bind and agglutinate red blood cells; this interaction is the basis of the direct hemagglutination assay, most often used to determine the titer of virus in solution.
- Neutralization assays quantify the level of virus-specific antibody by measuring the decrease in hemagglutination observed after mixing patient serum with a standardized amount of virus.
- Hemagglutination assays are also used to screen and cross-match donor and recipient blood to ensure that the transfusion recipient does not have antibodies to antigens in the donated blood.
Footnotes
- 1 Lancefield, Rebecca C., “The Antigenic Complex of Streptococcus haemoliticus. I. Demonstration of a Type-Specific Substance in Extracts of Streptococcus haemolyticus,” The Journal of Experimental Medicine 47, no. 1 (1928): 91-103.


