16.6: Superantigens
- Page ID
- 3383
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Define superantigen.
- Briefly describe the mechanism by which superantigens cause harm to the body.
- Name 2 superantigens and give an example of a bacterium that produces each.
As was learned earlier under Bacterial Pathogenicity, superantigens are type I toxins that can trigger a harmful immune response. Exotoxins are toxins, often proteins in nature, secreted from a living bacterium but also released upon bacterial lysis. In addition, some bacteria use a type 3 secretion system or a type 4 secretion system to inject toxins directly into human cells. There are three main types of exotoxins:
- Superantigens (Type I toxins),
- Exotoxins that damage host cell membranes (Type II toxins)
- A-B toxins and other toxin that interfere with host cell function (TypeIII toxins).
We will look at superantigens and their role in hypersensitivity.
- Define superantigen.
- Briefly describe the mechanism by which superantigens cause harm to the body.
- Name 2 superantigens and give an example of a bacterium that produces each.
Highlighted Bacterium
- Read the description of Streptococcus pyogenes and match the bacterium with the description of the organism and the infection it causes.
Superantigens are unusual bacterial toxins that interact with exceedingly large numbers of T4-lymphocytes. They bind to the surface of the target cell but do not enter the cell.
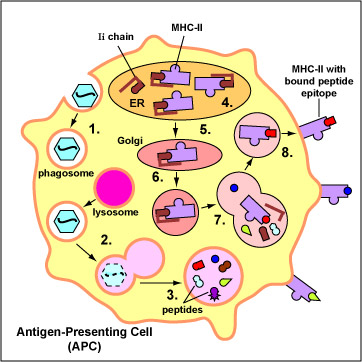
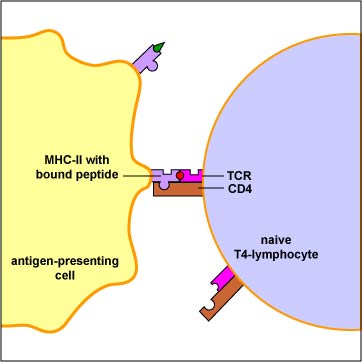
Conventional antigens are engulfed by antigen presenting cells (APCs), degraded into epitopes, bind to the peptide groove of MHC-II molecules, and are put on the surface of the APC (Figure \(\PageIndex{1}\)). Here they are recognized by specific T4-lymphocytes having a TCR with a corresponding shape (Figure \(\PageIndex{2}\)).
Superantigens, however, bind directly to the outside of MHC-II molecules and activate large numbers of T4-lymphocytes (Figure \(\PageIndex{3}\)). This activation of very large numbers of T4-lymphocytes results in the secretion of excessive amounts of a cytokine called interleukin-2 (IL-2) as well as the activation of self-reactive T-lymphocytes. The normal response to a conventional antigen results in the activation of maybe 1 in 10,000 T-lymphocytes; superantigens can activate as many as 1 in 5 T-lymphocytes.
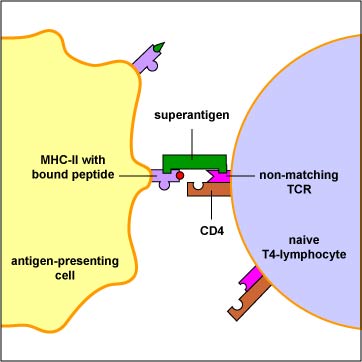
Production of high levels of IL-2 can result in circulation of IL-2 in the blood leading to symptoms such as fever, nausea, vomiting, diarrhea, and malaise. However, excess stimulation of IL-2 secretion can also lead to production of inflammatory cytokines such as tumor necrosis factor-alpha (TNF-alpha), interleukin-1 (IL-1), inflammatory chemokines such as IL-8, and platelet-activating factor (PAF), and can lead to the same endothelial damage, acute respiratory distress syndrome, disseminated intravascular coagulation, shock, and multiple organ system failure seen above with LPS and other bacterial cell wall factors. Activation of self-reactive T-lymphocytes can also lead to autoimmune attack.
The following are examples of superantigens.
- Toxic shock syndrome toxin-1 (TSST-1), produced by some strains of Staphylococcus aureus. This exotoxin causes toxic shock syndrome (TSS). Excessive cytokine production leads to fever, rash, and shock.
- Streptococcal pyrogenic exotoxin (Spe), produced by rare invasive strains and scarlet fever strains of Streptococcus pyogenes (the group A beta streptococci). S pyogenes produces a number of SPEs that are cytotoxic, pyrogenic, enhance the lethal effects of endotoxins, and contribute to cytokine-induced inflammatory damage. SPEs are responsible for causing streptococcal toxic shock syndrome (STSS) whereby excessive cytokine production leads to fever, rash, and triggering the shock cascade. The SPEs also appear to be responsible for inducing necrotizing fasciitis, a disease that can destroy the skin, fat, and tissue covering the muscle (the fascia). SPE B is also a precursor for a cysteine protease that can destroy muscles tissue.
Read the description of Streptococcus pyogenes, and be able to match the bacterium with its description on an exam.
- Staphylococcal enterotoxins (SE), producedby many strains of Staphylococcus aureus. These exotoxins cause staphylococcal food poisoning. Excessive Il-2 production results in fever, nausea, vomiting,and diarrhea. The vomiting may also be due to these toxins stimulating the vagus nerve in the stomach lining that controls vomiting.
- ETEC enterotoxin, produced by enterotoxogenic E. coli (ETEC), one of the most common causes of traveler's diarrhea.
What is the mechanism by which superantigens ultimately lead to SIRS?
Summary
- Conventional antigens are only recognized by specific T4-cells having a TCR with a corresponding shape.
- Superantigens are unusual bacterial toxins that interact with exceedingly large numbers of T4-lymphocytes.
- Activation of very large numbers of T4-lymphocytes results in the secretion of excessive amounts of a cytokine called interleukin-2 (IL-2).
- Excess stimulation of IL-2 secretion can also lead to production of inflammatory and can lead to the same endothelial damage, acute respiratory distress syndrome, disseminated intravascular coagulation, shock, and multiple organ system failure seen with PAMP-induced inflammation.
- Examples of superantigens include toxic shock syndrome toxin-1 (TSST-1), Streptococcal pyrogenic exotoxins (SPE), Staphylococcal enterotoxins (SE), and enterotoxogenic E. coli (ETEC) enterotoxin.
Questions
Study the material in this section and then write out the answers to these questions. Do not just click on the answers and write them out. This will not test your understanding of this tutorial.
- Define superantigen (ans).
- Briefly describe the mechanism by which superantigens cause harm to the body. (ans)
- Name 2 superantigens and give an example of a bacterium that produces each.
- (ans)
- (ans)
- Multiple Choice (ans)


