3.6: Lipids
- Page ID
- 16730
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)It glistens with fat, from the cheese to the steak. You may never have visited Philadelphia, but you probably know about its famous gastronomic delight, the Philly cheesesteak, pictured here. Both cheese and steak are typically high-fat foods, so this sandwich is definitely not recommended if you are following a low-fat diet. We need some fats in our diet for good health, but too much of a good thing can be harmful to our health, no matter how good it tastes. What are fats? And why do we have such a love-hate relationship with them? Read on to find out.

Lipids and Fatty Acids
Fats are actually a type of lipid. Lipids are a major class of biochemical compounds that includes oils as well as fats. Organisms use lipids to store energy and for many other uses.
Lipid molecules consist mainly of repeating units called fatty acids. There are two types of fatty acids: saturated fatty acids and unsaturated fatty acids. Both types consist mainly of simple chains of carbon atoms bonded to one another and to hydrogen atoms. The two types of fatty acids differ in how many hydrogen atoms they contain.
Saturated Fatty Acids
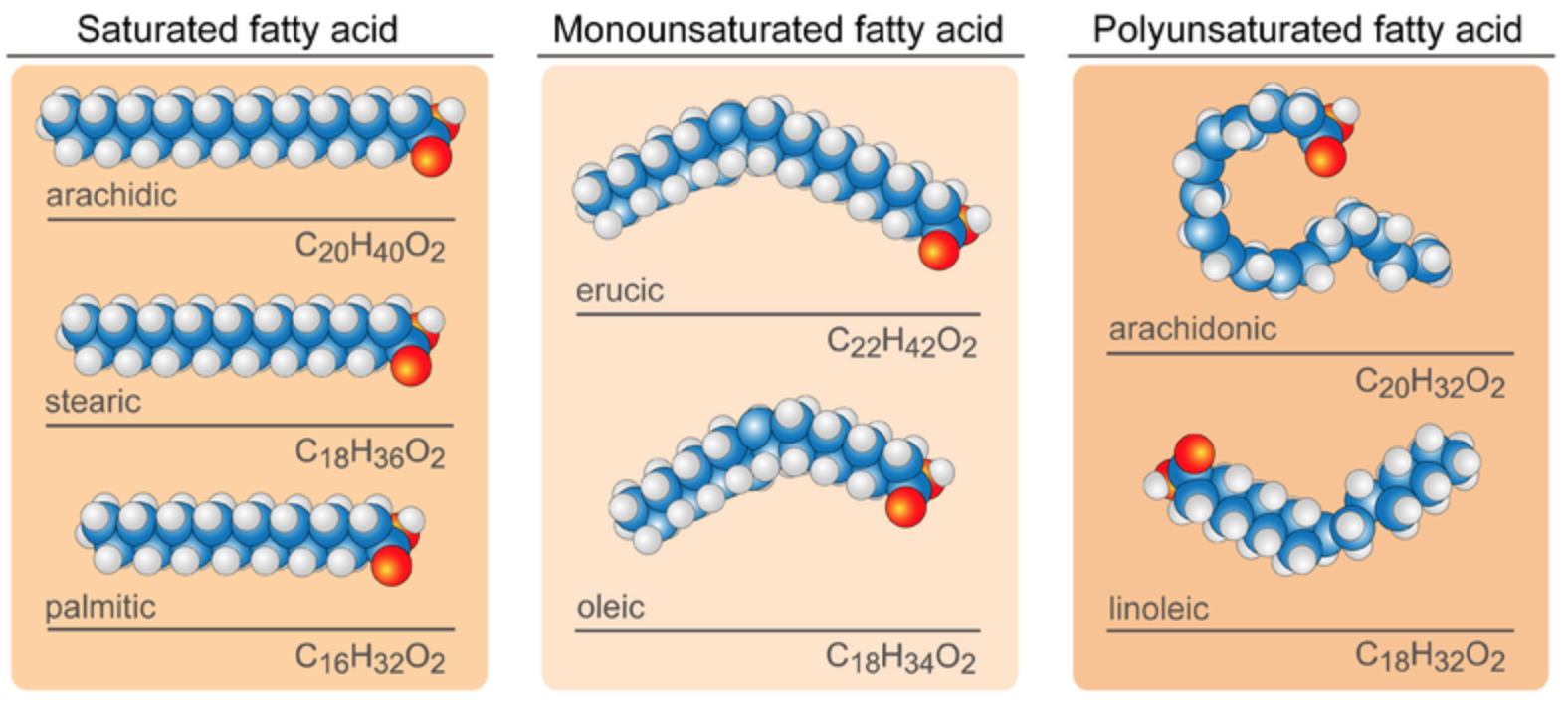
In saturated fatty acids, carbon atoms are bonded to as many hydrogen atoms as possible. All the carbon-to-carbon atoms share just single bonds between them. This causes the molecules to form straight chains, as shown in Figure \(\PageIndex{2}\). The straight chains can be packed together very tightly, allowing them to store energy in a compact form. Saturated fatty acids have relatively high melting points, explaining why they are solids at room temperature. Animals use saturated fatty acids to store energy.
Unsaturated Fatty Acids
In unsaturated fatty acids, some carbon atoms are not bonded to as many hydrogen atoms as possible. Instead, they form double or even triple bonds with other carbon atoms. This causes the chains to bend (see Figure \(\PageIndex{2}\)). The bent chains cannot be packed together very tightly. Unsaturated fatty acids have relatively low melting points, which explains why they are liquids at room temperature. Plants use unsaturated fatty acids to store energy.
Monounsaturated fatty acids contain one less hydrogen atom than the same-length saturated fatty acid chain. Monounsaturated fatty acids are liquids at room temperature but start to solidify at refrigerator temperatures. Good food sources of monounsaturated fats include olive and peanut oils and avocados.
Polyunsaturated fatty acids contain at least two fewer hydrogen atoms than the same-length saturated fatty acid chain. Polyunsaturated fatty acids are liquids at room temperature and remain in the liquid state in the refrigerator. Good food sources of polyunsaturated fats include safflower and soybean oils and many nuts and seeds.
Types of Lipids
Lipids may consist of fatty acids alone, or they may contain other molecules as well. For example, some lipids contain alcohol or phosphate groups. Types of lipids include triglycerides, phospholipids, and steroids. Each type has different functions in living things.
Triglycerides
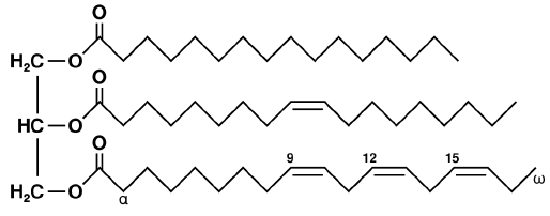
Triglycerides are formed by combining a molecule of glycerol with three fatty acid molecules. Glycerol (also called glycerine) is a simple compound known as a sugar alcohol. It is a colorless, odorless liquid that is sweet tasting and nontoxic. Triglycerides are the main constituent of body fat in humans and other animals. They are also found in fats derived from plants. There are many different types of triglycerides, with the main division being between those that contain saturated fatty acids and those that contain unsaturated fatty acids.
In the human bloodstream, triglycerides play an important role in metabolism as energy sources and transporters of dietary fat. They contain more than twice as much energy as carbohydrates, the other major source of energy in the diet. When you eat, your body converts any calories it doesn't need to use right away into triglycerides, which are stored in your fat cells. When you need energy between meals, hormones trigger the release of some of these stored triglycerides back into the bloodstream.
Phospholipids
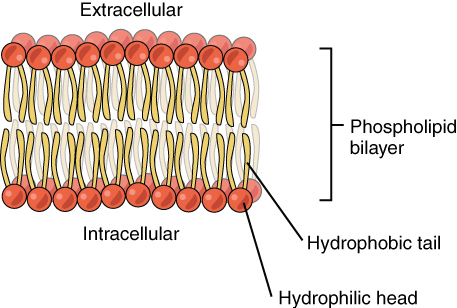
Phospholipids are a major component of the cell membranes of all living things. Each phospholipid molecule has a "tail" consisting of two long fatty acids and a "head" consisting of a phosphate group and glycerol molecule (see diagram below). The phosphate group is a small negatively charged molecule. The phospholipid head is hydrophilic or attracted to water. The fatty acid tail of the phospholipid is hydrophobic or repelled by water. These properties allow phospholipids to form a two-layer, or bilayer, cell membrane.
As shown in the diagram below, a phospholipid bilayer forms when many phospholipid molecules line up tail to tail, forming an inner and outer surface of hydrophilic heads. The hydrophilic heads point toward both the watery extracellular space and the watery intracellular space (lumen) of the cell.
Steroids
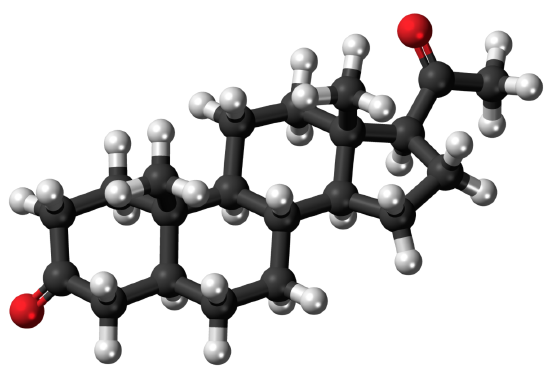
Steroids are lipids with a ring structure. Each steroid has a core of seventeen carbon atoms arranged in four rings of five or six carbons each (see model pictured below). Steroids vary by the other components attached to this four-ring core. Hundreds of steroids are found in plants, animals, and fungi, but most steroids have one of just two principal biological functions: some steroids, such as cholesterol, are important components of cell membranes; many other steroids are hormones, which are messenger molecules. In humans, steroid hormones include cortisone, a fight-or-flight hormone; and the sex hormones estrogen and testosterone.
During a routine checkup with your family doctor, your blood was collected for a lipid profile. The results are back, and your triglyceride level is 180 mg/dL. Your doctor says this is a little high. A blood triglyceride level of 150 mg/dL or lower is considered normal. Higher levels of triglycerides in the blood have been linked to increased risk of atherosclerosis, heart disease, and stroke.
If a blood test reveals that you have high triglycerides, the levels can be lowered through healthy lifestyle choices and/or prescription medications. Healthy lifestyle choices to control triglyceride levels include:
- losing weight. If you are overweight, losing even 5 or 10 pounds may help lower your triglyceride level.
- cutting back on calories. Extra calories are converted to triglycerides and stored as fat, so reducing your calories should also reduce your triglyceride level.
- avoiding sugary and refined foods. Simple carbohydrates, such as sugars and foods made with white flour, can increase triglyceride levels.
- choosing healthier fats. Trade saturated fats found in animal foods for healthier unsaturated fats found in plants and oily fish. For example, substitute olive oil for butter and salmon for red meat.
- limiting alcohol consumption. Alcohol is high in calories and sugar and has a strong effect on triglyceride levels.
- exercising regularly. Aim for at least 30 minutes of physical activity on most or all days of the week to lower triglyceride levels.
If healthy lifestyle changes aren't enough to bring down high triglyceride levels, drugs prescribed by your doctor are likely to help.
Review
- What are lipids?
- Compare and contrast saturated and unsaturated fatty acids.
- Identify three major types of lipids, and describe differences in their structures.
- How do triglycerides play an important role in human metabolism?
- Explain how phospholipids form cell membranes.
- What is cholesterol, and what is its major function?
- Give three examples of steroid hormones in humans.
- Which type of fatty acid do you think is predominant in the steak and cheese of the cheesesteak shown above? Explain your answer.
- Which type of fat would be the most likely to stay liquid in colder temperatures — bacon fat, olive oil, or soybean oil? Explain your answer.
- Why do you think that the shape of the different types of fatty acid molecules affects how easily they solidify?
- High cholesterol levels in the bloodstream can cause negative health effects but explain why we wouldn’t want to get rid of all the cholesterol in our bodies.
- Name two types of lipids that are part of the cell membrane.
- True or False. Fatty acids are made up of triglycerides.
- Which type of lipid often functions as chemical messenger molecules?
Explore More
Watch the video below to learn more about triglycerides and the difference between saturated and unsaturated fatty acids.
Attributions
- Philly cheesesteak by jeffreyw, licensed CC BY 2.0 via Wikimedia Commons
- Fatty acids by Mariana Ruiz Villarreal (LadyofHats), CC BY-NC 3.0 for CK-12 foundation
- Triglyceride by Wolfgang Schaefer, released into the public domain via Wikimedia Commons
- Phospholipid bilayer by OpenStax, CC BY 4.0 via Wikimedia Commons
- Steroid by Jynto, dedicated CC0 via Wikimedia Commons
- Text adapted from Human Biology by CK-12 licensed CC BY-NC 3.0


