3.5: Carbohydrates
- Page ID
- 16729
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Where would we be without our jeans? They have been the go-to pants for many people for decades, and they are still as popular as ever. Jeans are made of denim, a type of cotton fabric. Cotton is a soft, fluffy fiber that grows in a protective case around the seeds of cotton plants. The fiber is almost pure cellulose. Cellulose is the single most abundant biochemical compound found in Earth's living things and one of several types of carbohydrates.

What Are Carbohydrates?
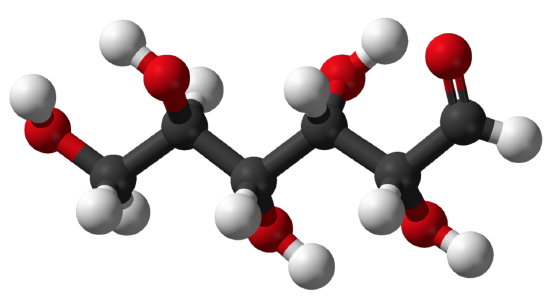
Carbohydrates are the most common class of biochemical compounds. They include sugars and starches. Carbohydrates are used to provide or store energy, among other uses. Like most biochemical compounds, carbohydrates are built of small repeating units, or monomers, which form bonds with each other to make larger molecules, called polymers. In the case of carbohydrates, the small repeating units are known as monosaccharides. Each monosaccharide consists of six carbon atoms, as shown in the model of the monosaccharide glucose below.
Sugars
Sugars are the general name for sweet, short-chain, soluble carbohydrates, which are found in many foods. Their function in living things is to provide energy. The simplest sugars consist of a single monosaccharide. They include glucose, fructose, and galactose. Glucose is a simple sugar that is used for energy by the cells of living things. Fructose is a simple sugar found in fruits, and galactose is a simple sugar found in milk.
Other sugars contain two monosaccharide molecules and are called disaccharides. An example is sucrose or table sugar. It is composed of one fructose molecule and one glucose molecule. Other disaccharides include maltose (two glucose molecules) and lactose (one glucose molecule and one galactose molecule). Lactose occurs naturally in milk. Some people can't digest lactose. If they drink milk, it causes gas, cramps, and other unpleasant symptoms unless the milk has been processed to remove the lactose.
Complex Carbohydrates
The simple sugars form the foundation of more complex carbohydrates. The cyclic forms of two sugars can be linked together by means of a condensation reaction. The figure below shows how a glucose molecule and a fructose molecule combine to form a sucrose molecule. A hydrogen atom from one molecule and a hydroxyl group from the other molecule are eliminated as water, with a resulting covalent bond linking the two sugars together at that point.
Glucose and fructose combine to produce the disaccharide sucrose in a condensation reaction as shown in Figure \(\PageIndex{3}\). Sucrose, commonly known as table sugar, is an example of a disaccharide.
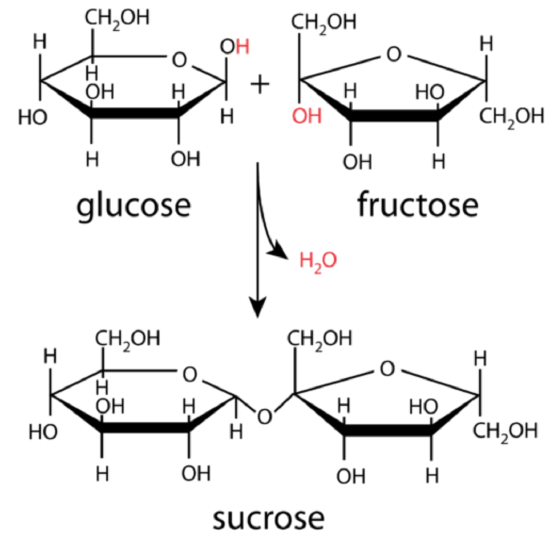
A disaccharide is a carbohydrate formed by the joining of two monosaccharides. Other common disaccharides include lactose and maltose. Lactose, a component of milk, is formed from glucose and galactose, while maltose formed from two glucose molecules. During digestion, these disaccharides are hydrolyzed in the small intestine to form the component monosaccharides, which are then absorbed across the intestinal wall and into the bloodstream to be transported to the cells.
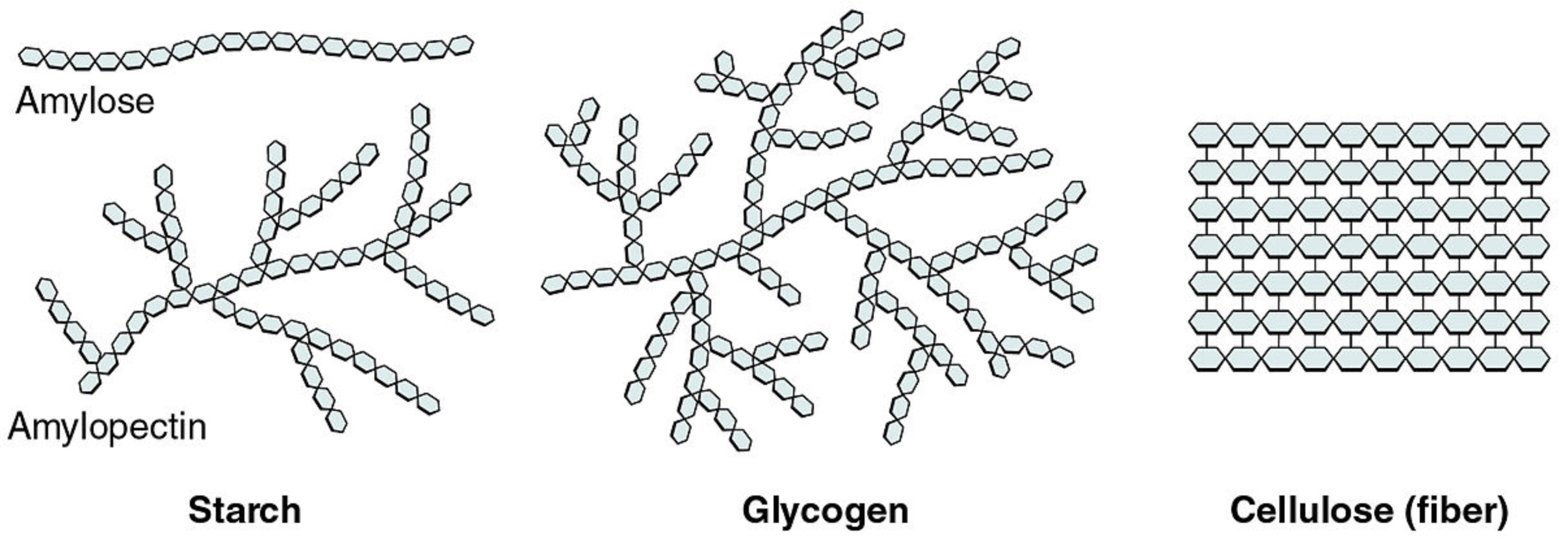
Some carbohydrates consist of hundreds or even thousands of monosaccharides bonded together in long chains. These carbohydrates are called polysaccharides ("many saccharides"). Polysaccharides are also referred to as complex carbohydrates. Complex carbohydrates that are found in living things include starch, glycogen, cellulose, and chitin. Each type of complex carbohydrate has different functions in living organisms but they generally either store energy or make up certain structures of living things.



Starch
Starch is a complex carbohydrate that is made by plants to store energy. For example, the potatoes pictured below are packed full of starches that consist mainly of repeating units of glucose and other simple sugars. The leaves of potato plants make sugars by photosynthesis, and the sugars are carried to underground tubers where they are stored as starch. When we eat starchy foods such as potatoes, the starches are broken down by our digestive system to sugars, which provide our cells with energy. Starches are easily and quickly digested with the help of digestive enzymes such as amylase, which is found in the saliva. If you chew a starchy saltine cracker for several minutes, you may start to taste the sugars released as the starch is digested.
Glycogen
Animals do not store energy as starch. Instead, animals store the extra energy as the complex carbohydrate glycogen. Glycogen is a polysaccharide of glucose. It serves as a form of energy storage in fungi as well as animals and is the main storage form of glucose in the human body. In humans, glycogen is made and stored primarily in the cells of the liver and the muscles. When energy is needed from either storage depot, the glycogen is broken down to glucose for use by cells. Muscle glycogen is converted to glucose for use by muscle cells, and liver glycogen is converted to glucose for use throughout the rest of the body. Glycogen forms an energy reserve that can be quickly mobilized to meet a sudden need for glucose, but one that is less compact than the energy reserves of lipids, which are the primary form of energy storage in animals.
Glycogen plays a critical part in the homeostasis of glucose levels in the blood. When blood glucose levels rise too high, excess glucose can be stored in the liver by converting it to glycogen. When glucose levels in the blood fall too low, glycogen in the liver can be broken down into glucose and released into the blood.
Cellulose
Cellulose is a polysaccharide consisting of a linear chain of several hundred to many thousands of linked glucose units. Cellulose is an important structural component of the cell walls of plants and many algae. Human uses of cellulose include the production of cardboard and paper, which consist mostly of cellulose from wood and cotton. The cotton fibers pictured below are about 90 percent cellulose.
Certain animals, including termites and ruminants such as cows, can digest cellulose with the help of microorganisms that live in their gut. Humans cannot digest cellulose, but it nonetheless plays an important role in our diet. It acts as a water-attracting bulking agent for feces in the digestive tract and is often referred to as "dietary fiber."
Chitin
Chitin is a long-chain polymer of a derivative of glucose. It is found in many living things. For example, it is a component of the cell walls of fungi, the exoskeletons of arthropods such as crustaceans and insects (including the beetle pictured in Figure \(\PageIndex{7}\)), and the beaks and internal shells of animals such as squids and octopuses. The structure of chitin is similar to that of cellulose.

You probably know that you should eat plenty of fiber, but do you know how much fiber you need, how fiber contributes to good health, or which foods are good sources of fiber? Dietary fiber consists mainly of cellulose, so it is found primarily in plant-based foods, including fruits, vegetables, whole grains, and legumes. Dietary fiber can't be broken down and absorbed by your digestive system. Instead, it passes relatively unchanged through your gastrointestinal tract and is excreted in feces. That's how it helps keep you healthy.
The fiber in food is commonly classified as either soluble or insoluble fiber.
- Soluble fiber dissolves in water to form a gel-like substance as it passes through the gastrointestinal tract. Its health benefits include lowering blood levels of cholesterol and glucose. Good sources of soluble fiber include whole oats, peas, beans, and apples.
- Insoluble fiber does not dissolve in water. This type of fiber increases the bulk of feces in the large intestine and helps keep food wastes moving through, which may help prevent or correct constipation. Good sources of insoluble fiber include whole wheat, wheat bran, beans, and potatoes.
How much fiber do you need for good health? That depends on your age and gender. The Institute of Medicine recommends the daily fiber intake for adults shown in the table below. Most dietitians further recommend a ratio of about 3 parts insoluble fiber to 1 part soluble fiber each day. Most fiber-rich foods contain both types of fiber, so it usually isn't necessary to keep track of the two types of fiber as long as your overall fiber intake is adequate.
Use food labels and online fiber counters to find out how much total fiber you eat in a typical day. Are you consuming enough fiber for good health? If not, consider ways to increase your intake of this important substance. For example, substitute whole grains for refined grains, eat more legumes such as beans, and try to consume at least five servings of fruits and vegetables each day.
| Gender | Age 50 or Younger | Age 51 or Older |
|---|---|---|
| Male | 38 grams | 30 grams |
| Female | 25 grams | 21 grams |
Summary
- Carbohydrates are the most common class of biochemical compounds. The basic building block of carbohydrates is the monosaccharide, which consists of six carbon atoms.
- Sugars are sweet, short-chain, soluble carbohydrates that are found in many foods and supply us with energy. Simple sugars, such as glucose, consist of just one monosaccharide. Some sugars, such as sucrose, or table sugar, consist of two monosaccharides and are called disaccharides.
- Complex carbohydrates, or polysaccharides, consist of hundreds or even thousands of monosaccharides. They include starch, glycogen, cellulose, and chitin. They generally either store energy or form structures, such as cell walls, in living things.
- Starch is a complex carbohydrate that is made by plants to store energy. Potatoes are a good food source of dietary starch, which is readily broken down to its component sugars during digestion.
- Glycogen is a complex carbohydrate that is made by animals and fungi to store energy. Glycogen plays a critical part in the homeostasis of blood glucose levels in humans.
- Cellulose is the single most common biochemical compound in living things. It forms the cell walls of plants and certain algae. Like most other animals, humans cannot digest cellulose, but it makes up most of the crucial dietary fiber in the human diet.
- Chitin is a complex carbohydrate, similar to cellulose, that makes up organic structures such as the cell walls of fungi and the exoskeletons of insects and other arthropods.
Review
- What are carbohydrates? Describe their structure.
- Compare and contrast sugars and complex carbohydrates.
- Identify the four main types of complex carbohydrates and their functions.
- If you chew on a starchy food such as a saltine cracker for several minutes, it may start to taste sweet. Explain why.
- True or False. Glucose is mainly stored by lipids in the human body.
- Put the following carbohydrates in order from smallest to largest: cellulose; fructose; sucrose
- Name three carbohydrates that contain glucose as a monomer.
- Jeans are made of tough, durable cotton. Explain how you think this fabric gets its tough qualities, based on what you know about the structure of carbohydrates.
- Which do you think is faster to digest — simple sugars or complex carbohydrates? Explain your answer.
- True or False. Cellulose is broken down in the human digestive system into glucose molecules.
- Which type of fiber dissolves in water? Which type does not dissolve in water?
- What are the similarities and differences between muscle glycogen and liver glycogen?
- Which carbohydrate is used directly by the cells of living things for energy?
- Which of the following is not a complex carbohydrate?
- chitin
- starch
- disaccharide
- none of the above
Explore More
Watch the video below to learn about the health impacts of carbohydrates.
Attributions
- Body paint by Cuerpos Pintados, licensed CC BY 2.0 via Wikimedia Commons
- Glucose public domain via Wikimedia Commons
- Sucrose by Christopher Auyeung and Joy Sheng, CC BY-NC 3.0, via CK-12
- Potatoes by Elza Fiuza/ABr, licensed CC BY 3.0 via Wikimedia Commons Brazil
- Cotton by KoS, released into the public domain via Wikimedia Commons
- Ten-lined June beetle by Junkyardsparkle, dedicated CC0 via Wikimedia Commons
- Three Polysaccharides by OpenStax College, licensed CC BY 3.0 via Wikimedia Commons Brazil
- Beans by Charles Brooking, released into the public domain via Wikimedia Commons
- Text adapted from Human Biology by CK-12 licensed CC BY-NC 3.0


