6.3: Some Details of Glycolysis
- Page ID
- 16445
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)A. Glycolysis, Stage 1
Reaction 1: In the first reaction of glycolysis, the enzyme hexokinase rapidly phosphorylates glucose entering the cell, forming glucose-6-phosphate (G-6-P). As shown below, the overall reaction is exergonic; the free energy change for the reaction is -4 Kcal per mole of G-6-P synthesized.

This is a coupled reaction, in which phosphorylation of glucose is coupled to ATP hydrolysis. The free energy of ATP hydrolysis (an energetically favorable reaction) fuels the glucose phosphorylation (an energetically unfavorable reaction). The reaction is also biologically irreversible, as shown by the single vertical arrow. Excess dietary glucose can be stored in most cells (especially liver and kidney cells) as a highly branched polymer of glucose monomers called glycogen. In green algae and plants, glucose made by photosynthesis is stored as polymers of starch. When glucose is necessary for energy, glycogen and starch hydrolysis forms glucose-1- phosphate (G-1-P) which is then converted to G-6-P.
Let’s look at the energetics (free energy flow) of the hexokinase-catalyzed reaction. This reaction can be seen as the sum of two reactions shown below.

Recall that ATP hydrolysis is an exergonic reaction, releasing ~7 Kcal/mole (rounding down!) in a closed system under standard conditions. The condensation reaction of glucose phosphorylation occurs with a DGo of +3 Kcal/mole. This is an endergonic reaction under standard conditions. Summing up the free energy changes of the two reactions, we can calculate the overall DGo of -4 Kcal/mole for the coupled reaction under standard conditions in a closed system.
The reactions above are written as if they are reversible. However, we said that the overall coupled reaction is biologically irreversible. Where’s the contradiction? To explain, we say that an enzyme-catalyzed reaction is biologically irreversible when the products have a relatively low affinity for the enzyme active site, making catalysis (acceleration) of the reverse reaction very inefficient. Enzymes catalyzing biologically irreversible reactions don’t allow going back to reactants, but they are often allosterically regulated. This is the case for hexokinase. Imagine a cell that slows its consumption of G-6-P because its energy needs are being met. What happens when G-6-P levels rise in cells? You might expect the hexokinase reaction to slow down so that the cell doesn’t unnecessarily consume a precious nutrient energy resource. The allosteric regulation of hexokinase is illustrated below.

As G-6-P concentrations rise in the cell, excess G-6-P binds to an allosteric site on hexokinase. The conformational change in the enzyme is then transferred to the active site, inhibiting the reaction.
152 Glycolysis Stage 1, Reaction 1
Reaction 2: In this slightly endergonic and reversible reaction, isomerase catalyzes the isomerization of G-6-P to fructose-6-P (F-6-P), as shown below.

Reaction 3: In this biologically irreversible reaction, 6-phosphofructokinase (6-P- fructokinase) catalyzes the phosphorylation of F-6-P to make fructose 1,6 di- phosphate (F1,6 diP). This is also a coupled reaction, in which ATP provides the second phosphate. The overall reaction is written as the sum of two reactions, as shown below.

Like the hexokinase reaction, the 6-P-fructokinase reaction is a coupled, exergonic and allosterically regulated reaction. Multiple allosteric effectors, including ATP, ADP and AMP and long-chain fatty acids regulate this enzyme.
Reactions 4 and 5: These are the last reactions of the first stage of glycolysis. In reaction 4, F1,6 diP (a 6-C sugar) is reversibly split into dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate (G-3-P). In reaction 5 (also reversible), DHAP is converted into another G-3-P. Here are the reactions:

The net result is the formation of two molecules of G-3-P in the last reactions of Stage 1 of glycolysis. The enzymes F-diP aldolase and triose-P-isomerase both catalyze freely reversible reactions. Also, both reactions proceed with a positive free energy change and are therefore endergonic. The sum of the free energy changes for the splitting of F1,6 diP into two G-3-Ps is a whopping +7.5 Kcal per mole, a very energetically unfavorable process.
Summing up, by the end of Stage 1 of glycolysis, we have consumed two ATP molecules, and split one 6C carbohydrate into two 3-C carbohydrates. We have also seen two biologically irreversible and allosterically regulated enzymes.
B. Glycolysis, Stage 2
We will follow just one of the two molecules of G-3-P generated by the end of Stage 1 of glycolysis, but remember that both are proceeding through Stage 2 of glycolysis.
Reaction 6: This is a redox reaction. G-3-P is oxidized to 1,3, diphosphoglyceric acid (1,3, diPG) and NAD+ is reduced to NADH. The reaction catalyzed by glyceraldehyde-3-phopsphate dehydrogenase is shown below.

In this freely reversible endergonic reaction, a hydrogen molecule (H2) is removed from G-3-P, leaving behind phosphoglyceric acid. This short-lived oxidation intermediate is phosphorylated to make 1,3 diphosphoglyceric acid (1,3diPG). At the same time, the hydrogen molecule is split into a hydride ion (H-) and a proton (H+). The H- ions reduce NAD+ to NADH, leaving the protons behind in solution. Remember that all of this is happening in the active site of the same enzyme!
Even though it catalyzes a reversible reaction, G-3-P dehydrogenase is allosterically regulated. However, in contrast to the regulation of hexokinase, that of G-3-P dehydrogenase is more complicated! The regulator is NAD+ and the mechanism of allosteric regulation of G-3-P dehydrogenase by NAD+ is called negative cooperativity. It turns out that the higher the [NAD+] in the cell, the lower the affinity of the enzyme for more NAD+ and the faster the reaction in the cell! The mechanism is discussed at the link below.
154 Glycolysis Stage 2; Reaction 6
Reaction 7: The reaction shown below is catalyzed by phosphoglycerate kinase. It is freely reversible and exergonic, yielding ATP and 3-phosphoglyceric acid (3PG).

Catalysis of phosphate group transfer between molecules by kinases is called substrate-level phosphorylation, often the phosphorylation of ADP to make ATP. In this coupled reaction the free energy released by hydrolyzing a phosphate from 1,3 diPG is used to make ATP. Remember that this reaction occurs twice per starting glucose. Two ATPs have been synthesized to this point in glycolysis. We call 1,3 diPG a very high-energy phosphate compound.
Reaction 8: This freely reversible endergonic reaction moves the phosphate from the number 3 carbon of 3PG to the number 2 carbon as shown below.
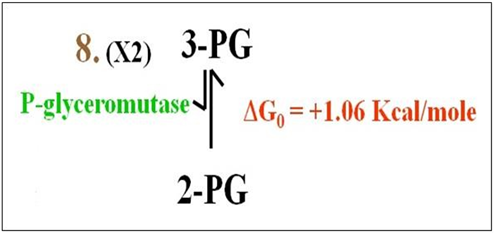
Mutases like phoshoglycerate mutase catalyze transfer of functional groups within a molecule.
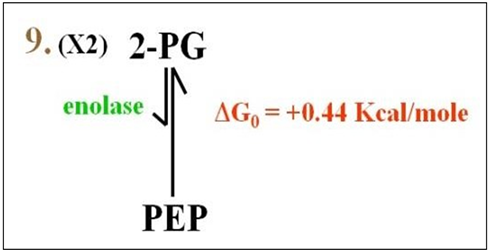
Reaction 9: In this reaction (shown below), enolase catalyzes the conversion of 2PG to phosphoenol pyruvate (PEP).
Reaction 10: This reaction results in the formation of pyruvic acid (pyruvate), as shown below. Remember again, two pyruvates are produced per starting glucose molecule.

The enzyme pyruvate kinase couples the biologically irreversible, exergonic hydrolysis of a phosphate from PEP and transfer of the phosphate to ADP in a coupled reaction. The reaction product, PEP, is another very high-energy phosphate compound.
155 Glycolysis Stage 2; Reactions 7-10
Pyruvate kinase is allosterically regulated by ATP, citric acid, long-chain fatty acids, F1,6 diP, and one of its own substrates, PEP.
In incomplete (aerobic) glycolysis, pyruvate is oxidized in mitochondria during respiration (see the Alternate Fates of Pyruvate above). Fermentations are called complete glycolysis because pyruvate is reduced to one or another end product. Recall that muscle fatigue results when skeletal muscle uses anaerobic fermentation to get energy during vigorous exercise. When pyruvate is reduced to lactic acid (lactate), lactic acid accumulation causes muscle fatigue. The enzyme Lactate Dehydrogenase (LDH) that catalyzes this reaction is regulated, but not allosterically. Instead different muscle tissues regulate LDH by making different versions of the enzyme! Click the Link below for an explanation.
156 Fermentation: Regulation of Pyruvate Reduction is NOT Allosteric!
C. A Chemical and Energy Balance Sheet for Glycolysis
Compare the balance sheets for complete glycolysis (fermentation) to lactic acid and incomplete (aerobic) glycolysis, showing chemical products and energy transfers.

There are two reactions in Stage 2 of glycolysis that each yield a molecule of ATP. Since each of these reactions occurs twice per starting glucose molecule, Stage 2 of glycolysis produces four ATP molecules. Since Stage 1 consumed two ATPs, the net yield of chemical energy as ATP by the end of glycolysis is two ATPs, whether complete to lactate or incomplete to pyruvate! Because they can’t make use of oxygen, anaerobes have to settle for the paltry 15 Kcal worth of ATP that they get from a fermentation. Since there are 687 Kcal potentially available from the complete combustion of a mole of glucose, there is a lot more free energy left to be captured during the rest of respiration.
157 Balance Sheet of Glycolysis
Remember also that the only redox reaction in aerobic glycolysis is in Stage 2. This is the oxidation of G-3-P, a 3C glycolytic intermediate. Now check out the redox reaction a fermentation pathway. Since pyruvate, also a 3C intermediate, was reduced, there has been no net oxidation of glucose (i.e., glycolytic intermediates) in complete glycolysis.
By this time, you will have realized that glycolysis is a net energetically favorable (downhill, spontaneous) reaction pathway in a closed system, with an overall negative ΔGo. Glycolysis is also normally spontaneous in most of our cells, driven by a constant need for energy to do cellular work. Thus the actual free energy of glycolysis, or ΔG’, is also negative. In fact, glycolysis in actively respiring cells proceeds with release of more free energy than it would in a closed system. In other words, the ΔG’ for glycolysis in active cells is more negative than the ΔGo of glycolysis!
Now, for a moment, let’s look at gluconeogenesis, the Atkins Diet and some not-so- normal circumstances when glycolysis essentially goes in reverse, at least in a few cell types. Under these conditions, glycolysis is energetically unfavorable, and those reverse reactions are the ones proceeding with a negative ΔG’!


