10.4: Recalls
- Page ID
- 39536
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Recalls - Drugs, Biologics, Devices, & Food
A recall is the voluntary removal of a product by a manufacturer or at the request of the FDA. Recalls are almost always voluntary. The FDA can only issue a recall when they have the mandated power to do so. The FDA cannot recall a drug, or biologic, but can recall a medical device, some cosmetics, and food. When they do have recall authority, they can only do so when there is a substantial public health and safety risk. https://www.fda.gov/consumers/consumer-updates/fda-101-product-recalls
Recall Resources: https://www.fda.gov/safety/recalls-m...call-resources
Food
The FDA can issue a food recall. In 2011, the FDA gained increased authority in regulating and responding to food product contamination via the new Food Safety Modernization Act (FSMA). The FSMA allows the FDA to suspend the services and production of food distributors if contamination is suspected. There need not be any proof of the source of the contamination. For more information on the FSMA: http://www.foodsafety.gov/news/fsma.html
Medical Device
The FDA can issue a device recall. In 2012, the US Food and Drug Administration (FDA) announced that it was seeking to implement medical device recall authority under § 518(e) of the FD&C Act and Chapter 21, Section 810 of the CFR. Recall authority for medical devices would permit the FDA to order manufacturers to cease the distribution of a device and notify health professionals if FDA finds a reasonable probability that the device would cause serious adverse health reactions or death (fda.gov). A recall does not necessarily mean the product must be returned, sometimes it just needs to be adjusted, or clarification safety instructions provided. 21 CFR 7 provides Guidance for conducting an efficient voluntary recall.
Examples of the types of actions that may be considered device recalls:
- Inspecting the device for problems
- Repairing the device
- Adjusting settings on the device
- Re-labeling the device
- Destroying device
- Notifying patients of a problem
- Monitoring patients for health issues
A medical device recall is either a correction or removal. A Correction addresses a problem with a medical device in the place where it is sold. A Removal approaches the problem by removing the device from where it is sold. In most cases, a company voluntarily recalls a device on its own. When the company has violated an FDA law, the company must recall the device (correction or removal) and notify the FDA. Legally, the FDA can require a company to recall a device if an organization refuses to do so under 21 CFR 810, Medical Device Recall Authority. 21 CFR 810 describes the procedures the FDA follows in exercising its medical device recall authority under section 518(e) of the FD&C Act.
A recall does not include a market withdrawal or a stock recovery. A market withdrawal is a firm's removal or correction of a distributed product which involves a minor violation that would not be subject to legal action by the FDA or which involves no violation, e.g., normal stock rotation practices, routine equipment adjustments, and repairs (fda.gov). In the end, almost all recalls are conducted voluntarily by the manufacturer. https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts
Explore!
A comprehensive, searchable recall medical device recall database: www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRES/res.cfm
To learn more about Device Recalls, visit the FDA device recall web page https://www.fda.gov/medicaldevices/safety/listofrecalls/ and also, watch the FDA Video here. http://fda.yorkcast.com/webcast/Play/1b95461f64be40ecbe3415195cb394911d
Device recalls following the same general recall procedure, as previously discussed for drugs. This includes classification of recall (I, II, or III) developing a recall strategy and providing the FDA with recall status reports.
Medical device safety alert: issued in situations where a medical device may present an unreasonable risk of substantial harm. In some cases, these situations also are considered recalls (fda.gov)
Market withdrawal occurs when a product has a minor violation that would not be subject to FDA legal action. The firm removes the product from the market or corrects the violation. For example, a product removed from the market due to tampering, without evidence of manufacturing or distribution problems would be a market withdrawal. (fda.gov)
Drug Safety and Availability
The FDA offers many websites that address specifically drug safety and availability to communicate with customers. Here are a few you may want to explore. https://www.fda.gov/drugs/drug-safety-and-availability
- Drug Safety Communications: https://www.fda.gov/drugs/drug-safety-and-availability/drug-safety-communications
- Post-Market Drug Safety: https://www.fda.gov/drugs/drug-safety-and-availability/postmarket-drug-safety-information-patients-and-providers
- What’s New – Human Drugs: https://www.fda.gov/drugs/news-events-human-drugs
- Adverse Event Reporting: https://www.fda.gov/drugs/surveillance/questions-and-answers-fdas-adverse-event-reporting-system-faers
Drug Recall
A drug recall is a voluntary action taken by a company. www.fda.gov/drugs/drug-recalls/fdas-roledrug-recalls. Not all recalls are announced on FDA.gov or in the news media. Public notification is generally issued when a product that has been widely distributed or poses a serious health hazard is recalled. However, if a company does not issue public notification of a recall, FDA may do so if the agency determines it is necessary to protect patients. FDA evaluates the effectiveness of a recall by evaluating a company’s efforts to properly notify customers and remove the defective product from the market. If a recall is determined to be ineffective FDA will request the company take additional actions.
Enforcement reports: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/enforcement-reports
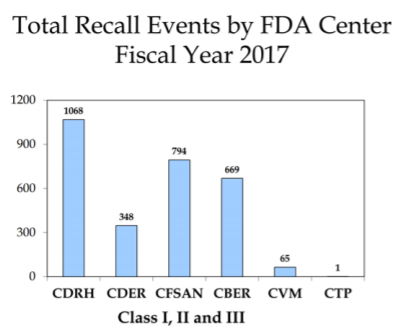
Class I, II & III Recalls
- Class I Recall: "A reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death." (fda.gov)
- Class II Recall: "use of or exposure to a violative product may cause temporary or medically reversible adverse health consequences or where the probability of serious adverse health consequences is remote." (fda.gov)
- Class III Recall: "use of or exposure to a violative product is not likely to cause adverse health consequences." (fda.gov)
Test Your Knowledge!
Read the article and watch the video “FDA 101: Product Recalls - From First Alert to Effectiveness Checks” and read FDA FAQs:
- How does the FDA first learn about a problem with a product?
- How does the FDA alert the product about a product recall?
- Discuss an example of a recall, and state what class of recall it is and why.
Explore!
The FDA Drug Safety Podcasts are produced by FDA's CDER and provide emerging safety information about drugs in conjunction with the release of Public Health Advisories and other drug safety issues. (fda.gov).


