2.7: Carbohydrates
- Page ID
- 24738
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Introduction: Carbohydrates
Carbohydrates serve 2 major functions: energy and structure. As energy, they can be simple for fast utilization or complex for storage. Simple sugars are monomers called monosaccharides. These are readily taken into cells and used immediately for energy. The most important monosaccharide is glucose (C6H12O6), since it is the preferred energy source for cells. The conversion of this chemical into cellular energy can be described by the equation below:
C6H12O6 (s) + 6 O2 (g) → 6 CO2 (g) + 6 H2O (l) + energy
Long polymers of carbohydrates are called polysaccharides and are not readily taken into cells for use as energy. These are used often for energy storage. Examples of energy storage molecules are amylose, or starch, (plants) and glycogen (animals). Some polysaccharides are so long and complex that they are used for structures like cellulose in the cell walls of plants. Cellulose is very large and practically indigestible, making it unsuitable as a readily available energy source for cells.
Carbohydrates: Carbohydrates are composed of sugar units referred to as -saccharides.
Many monosaccharides such as glucose and fructose are reducing sugars, meaning that they possess free aldehyde or ketone groups that reduce weak oxidizing agents such as the copper in Benedict’s reagent. The double bond in the carbonyl group is a source of electrons that can be donated to something else. That is to say, those electrons can be “lost” by the sugar and “gained” by another chemical. Benedict’s reagent contains cupric (copper) ion complexed with citrate in alkaline solution. Benedict’s test identifies reducing sugars based on their ability to reduce the cupric (Cu2+) ions to cuprous oxide (Cu+) at basic (high) pH. Cuprous oxide is green to reddish-orange. Roughly speaking, reduction is a type of chemical reaction that is paired with oxidation. In oxidation/reduction reactions (RedOx), some chemical loses electrons (oxidized) to another chemical that gains them (reduced). We remember whether a compound is reduced or gained by using the pneumonic: LEO goes GER or Loss of Electrons is Oxidation & Gain of Electrons is Reduction.
Monosaccharides contain a carbonyl group. The carbonyl is a source of electrons (the double bond on the oxygen). These electrons can be donated (or lost and oxidized) to reduce another compound (that gains those electrons).
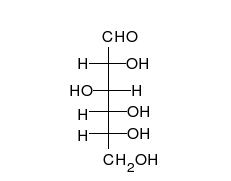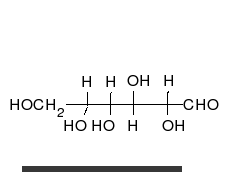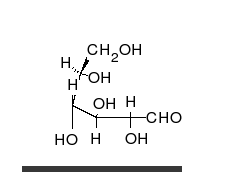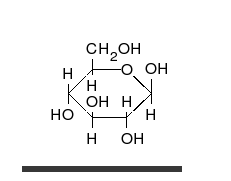
Glucose is the preferred carbohydrate of cells. In solution, it can change from a linear chain to a ring.
Monosaccharides are capable of isomerizing. This means they alternate in structure from a linear chain to a ring form in solution. In the chain form, the aldehyde is free to donate (lose) electrons to reduce another compound. When monosaccharides undergo dehydration synthesis to form polymers, they can no longer isomerize into chains with free aldehydes and are unable to act as reducing sugars. Green color indicates a small amount of reducing sugars, and reddish-orange color indicates an abundance of reducing sugars. Non-reducing sugars produce no change in color (i.e., the solution remains blue).
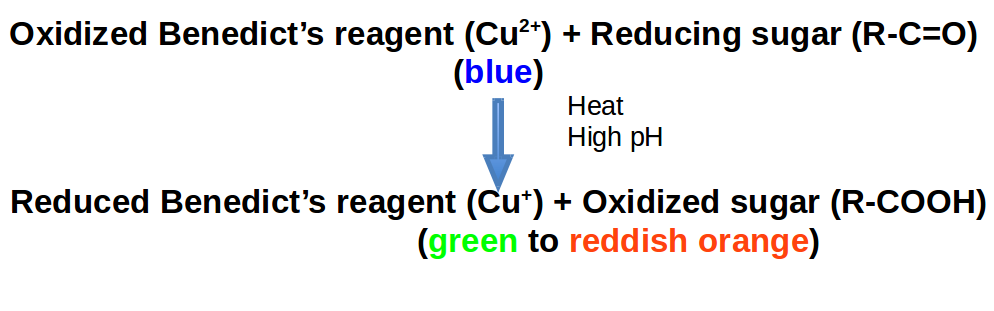
Note: Cu2+ has fewer electrons than Cu+.
When monosaccharides undergo dehydration synthesis to form polymers, they can no longer isomerize into chains with free aldehydes and are unable to act as reducing sugars. Green color indicates a small amount of reducing sugars and reddish-orange color indicates an abundance of reducing sugars. Non-reducing sugars produce no change in color (i.e., the solution remains blue).
Structural Carbohydrates
In food, more complex carbohydrates are derived from larger polysaccharides. These larger carbohydrates are fairly insoluble in water. Dietary fiber is the name given to indigestible materials in food most often derived from the complex carbohydrates from vegetable material. Some of this material serves the plants as a structural component of the cells and is completely insoluble. Cellulose is the major structural carbohydrate found in plant cell walls. Similarly, animals and fungi have structural carbohydrates that are composed of the indigestible compound called chitin. We will not be testing for these items.
Cellulose is a complex carbohydrate of glucose molecules. It is the major structural component of plant cell walls. It’ structural durability is enhanced by intramolecular hydrogen bonds.
Chitin is a structural carbohydrate found in animal shells or fungi cell walls. The polymer contains amide groups that differentiate it from other carbohydrates composed of glucose.

A cicada molting from its shell made of chitin.
Test Your Knowledge
Detection of Carbohydrates (Activity)
Materials:
|
|
Stop and Think
- Use your senses and previous observations/experiences about the qualities of the experimentals.
- Formulate some hypotheses about the carbohydrate content of the experimentals or unknowns.
- Identify if the sample is experimental or control before making a hypothesis.
Question: Are There Simple Reducing Sugars in my Juice? Are There Simple Reducing Sugars in my Urine?
Diabetes mellitus is a disease that refers to the inability of the cells to take in glucose. The word diabetes refers to urination and mellitus refers to sweetness. Since the cells of diabetics cannot remove glucose from the blood, there is an excess of glucose circulating that is eliminated in the urine. The traditional method of diagnosing someone with diabetes mellitus was to taste the sweetness of the patient’s urine. Let’s use Benedict’s test for the detection process instead of the unhygienic alternative.
Make a hypothesis and ask what we would predict from a Benedict’s test if testing a urine sample of someone with diabetes mellitus.
Benedict’s Test For Reducing Sugars
- Obtain 9 test-tubes and number them 1-9.
- Add to each tube the materials to be tested. Your instructor may ask you to test some additional materials. If so, include additional numbered test tubes.
- Indicate in the table whether the sample you are testing is positive control, a negative control, or an experimental.
- Before you begin the heating of the samples, predict the color change (if any) for each sample. (use the sample type to aid in your prediction)
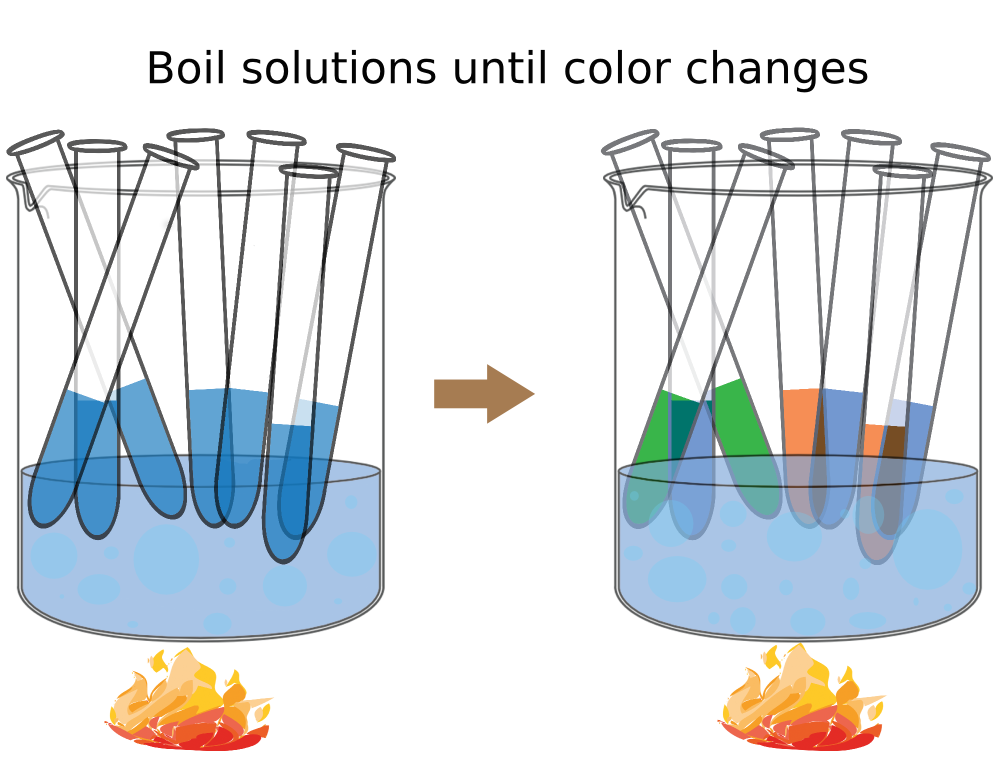
- Add 40 drops (or 2 ml) Benedict’s solution to each tube.
- Place all of the tubes in a boiling water bath for 3 min or until a noticeable color change and observe colors during this time.
- After 3 minutes, remove the tubes from the water bath and let them cool to room temperature. Record the color of their contents in the Table.
Iodine Test for Starch
Carbohydrates that are used for energy storage are not reducing sugars since they are polymers that lack free aldehydes. Plant cells store energy in the form of starches like amylose or pectin. Since these molecules are larger than monosaccharides or disaccharides, they are not sweet to the taste and are not very soluble in water. Iodine (iodine-potassium iodide, I2KI) staining distinguishes starch from monosaccharides, disaccharides, and other polysaccharides. The basis for this test is that starch is a coiled polymer of glucose — iodine interacts with these coiled molecules and becomes bluish-black. Iodine does not react with other carbohydrates that are not coiled and remains yellowish brown. Therefore, a bluish-black color is a positive test for starch, and a yellowish-brown color (i.e., no color change) is a negative test for starch. Notably, glycogen, a common energy storage polysaccharide in animals, has a slightly different structure than does starch and produces only an intermediate color reaction.
Plants store carbohydrates as a simple repeating polymer of glucose called starch. Amylose is a type of starch. Animal cells store glucose into a storage polymer called glycogen which is slightly more complicated than amylose.
Activity: Iodine Test For Starch
- Obtain 7 test-tubes and number them 1-7.
- Hypothesis Testing: Indicate in the table if the sample is experimental or control. Predict your expected color changes for each sample.
- Add to each tube the materials to be tested as indicated in the table below. Your instructor may ask you to test some additional materials. If so, include additional numbered test tubes.
- Add 10 drops of iodine to each tube. This test does NOT require boiling.
- Record the color of the tubes’ contents in the table below.
Questions for Reflection
1. In the Benedict’s test, which of the solutions is a positive control? Which is a negative control?
2. Which is a reducing sugar, sucrose or glucose?
3. Which contains more reducing sugars, potato juice or onion juice?
4. Is there a difference between the storage of sugars in onions and potatoes?
5. Which patient sample likely comes from a diabetic patient and how do we know this?
6. In the Iodine test, which of the solutions is a positive control? Which is a negative control?
7. Which is more positive for the iodine test: onion juice or potato juice?
8. What can you infer about the storage of carbohydrates in onions? In potatoes?
9. Describe the half-reaction Cu2+ → Cu+ as oxidation or reduction.
10. Describe the half-reaction Cu+ →Cu as oxidation or reduction.


