15.4: Regulation of Glycolysis
- Page ID
- 77734
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)There are three major enzymatic control points within the glycolytic pathway. These include hexokinase, phosphofructokinase, and pyruvate kinase reactions. Key drivers for regulating the pathway are energy demand:
- within the cell as determined by local indicators such as ATP and AMP
- within the organism as a whole, which can be influenced by hormone signaling pathways.
We will also see that the regulation of the pathway can vary depending on cell type and cellular needs.
Here is quick review of some key hormonal effects before we proceed.
Under low serum glucose conditions
- glucagon is secreted. This turns on glucose synthesis and glycogen breakdown and inhibits glycolysis in the liver. Glucose is then exported into the blood and restores higher glucose concentration.
Under high serum glucose
- insulin is secreted which promotes glucose uptake in the liver and muscle for energy use (glycolysis is activated and gluconeogenesis is inhibited) and storage (glycogen synthase is activated).
Hexokinase Regulation
One of the primary mechanisms that control the regulation of the hexokinase step in glycolysis is the presence of different hexokinase enzymes in different cellular types. Essentially, these are proteins that are encoded by different genes but perform the same function within the cell. They are known as isozymes. Isozymes can have different enzyme kinetics, different expression patterns in different tissues, different post-translational modifications, and bind with different allosteric effectors. This affords the body to have differential control over the same processes in different locations within the body. Within vertebrates, four important hexokinase isozymes vary in subcellular locations and kinetics parameters. This allows the differential phosphorylation of hexoses depending on local conditions, and physiological function. They are designated hexokinases I, II, III, and IV. All of the Hexokinases can use multiple hexoses as substrates, in addition to glucose. These include mannose, fructose, and 2-deoxyglucose. Hexokinase IV is also often referred to as Glucokinase and is specific to the Liver and Pancreas.
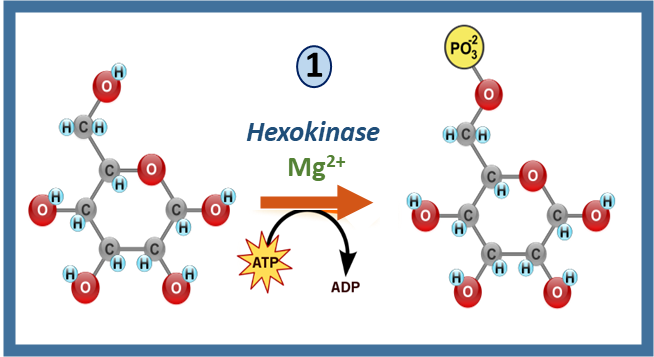
Recall that hexokinase enzymes mediate the first step in the glycolytic pathway with the formation of glucose 6-phosphate, as shown in Figure \(\PageIndex{1}\) below. They also require ATP as a cofactor in the process.
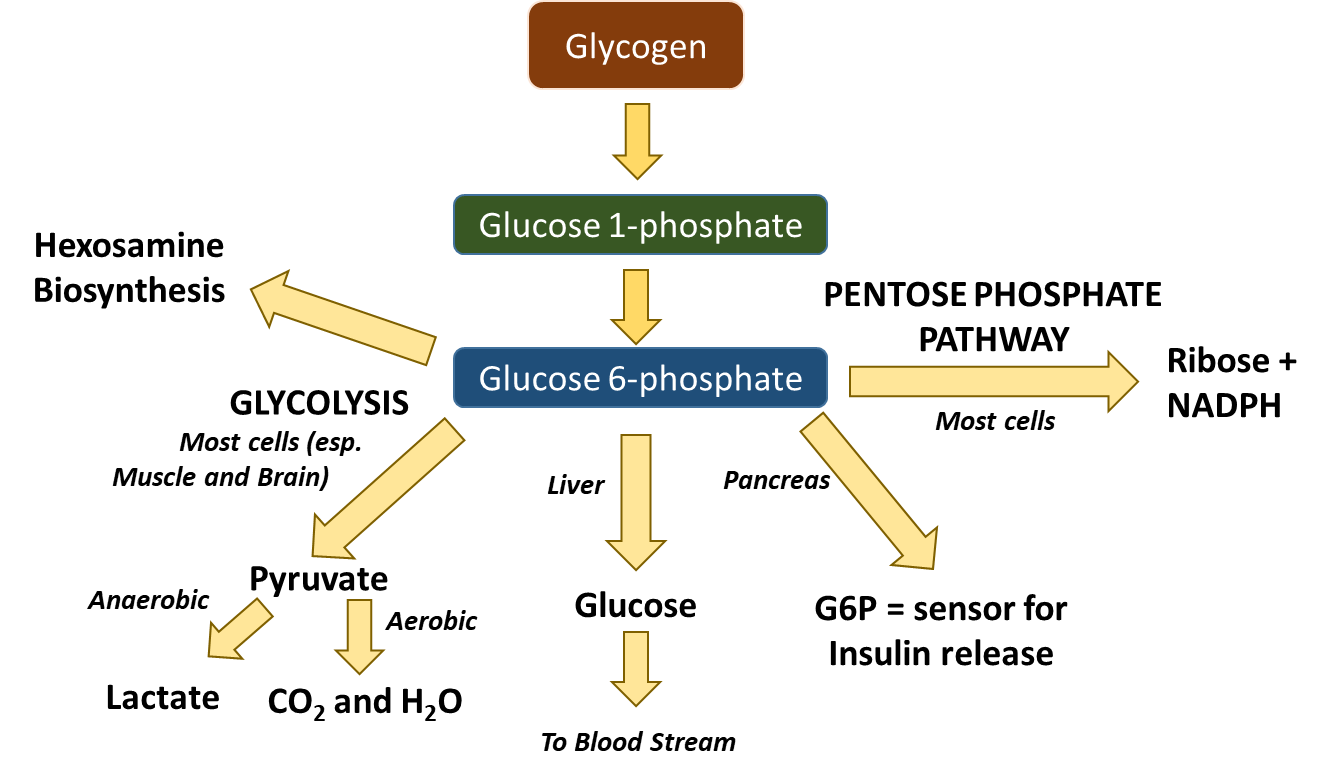
Recall that glucose-6-phosphate (G6P) has several potential fates within the body, as shown in Figure \(\PageIndex{2}\). It can be used as an energy source through the pathways of glycolysis and aerobic respiration. Short bursts of anaerobic respiration can also be sustained in animals that convert pyruvate into lactate. G6P can also be dephosphorylated in the liver and released back into the bloodstream to maintain homeostatic balance. The pancreas uses G6P as a sensor to determine when to secrete insulin and glucagon. The G6P can also serve as a building block for anabolic processes. It can be converted to ribose through the Pentose Phosphate Pathway where it will be used in the construction of nucleotide monomers. It can also be used for the formation of hexosamines used in proteoglycan and glycoprotein formation.
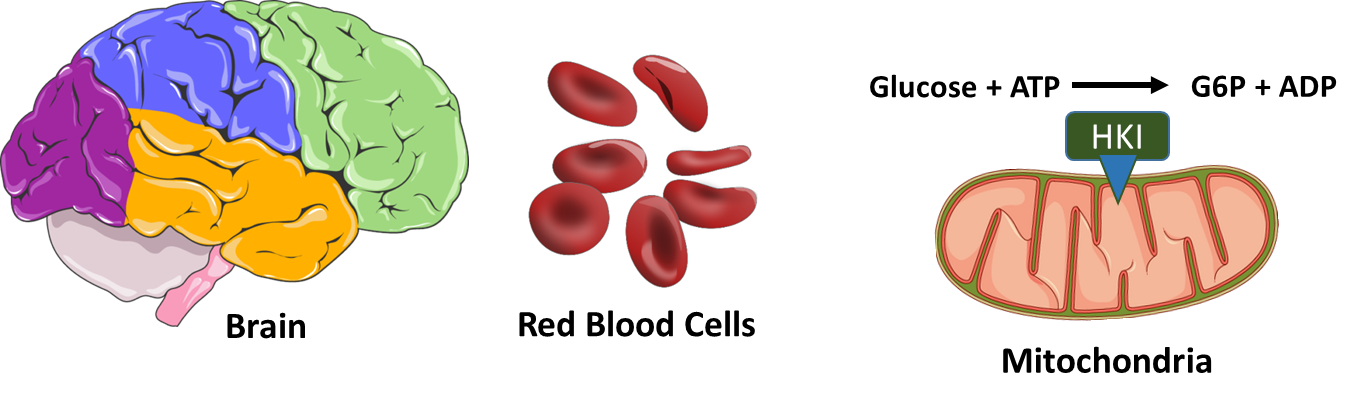
Now let’s take a look at the different isozymes of hexokinase in a little more detail. Hexokinase I (HKI) is found widely distributed throughout the body and is the main form expressed in brain tissue and red blood cells, as shown in Figure \(\PageIndex{3}\). In brain cells, this protein is localized to the mitochondria. This colocalization aids in the efficient coupling of glycolysis and the Krebs' cycle and oxidative phosphorylation pathways inside the mitochondria. It also ties the activity of HKI with oxidative phosphorylation and energy load, as HKI preferentially uses mitochondrially-derived ATP in its reaction mechanism. HK association with the mitochondria also has a cellular protective effect, reducing the potential for programmed cell death or apoptosis to occur. Red Blood Cells (RBCs), on the other hand, are highly differentiated cells with a very short lifespan. They are replaced in humans approximately every two weeks. RBCs are enucleated and do not have mitochondria, and thus, only generate ATP through the process of glycolysis. the HKI protein is free floating in the cytoplasm in this system. HKI has a low Km, meaning that it has a high affinity for glucose and is active at low substrate concentrations. It is also inhibited by the product Glucose-6-Phosphate (G6P) in the process of negative feedback inhibition. Essentially, you do not want to waste time and energy making more than you need. Low to moderate levels of free inorganic phosphate can overcome this negative feedback inhibition.
HKI and HKII are expressed in Skeletal Muscle, Heart Muscle, and insulin-sensitive tissues. While it is thought that HKI is providing a predominantly catabolic role for the use of G6P in energy production, HKII may play a more pertinent role in anabolic processes, providing G6P for conversion to G1P and subsequent utilization in Glycogenesis. Both HKI and HKII are localized to the outer membrane of the mitochondria. However, while 95% of HKI is associated with mitochondria, only about 70% of HKII is associated, with the remaining HKII fractionating with the cytosolic proteins. This could help to explain the heightened role of HKII in anabolic glycogenesis processes within skeletal muscle, and why it is not found in brain tissue. HKII is also often overexpressed in tumor cells, where it is associated with higher mortality rates. It has also been linked with the processes of metastasis and with the development of drug resistance. Similar to HKI, HKII also has a low Km and is inhibited by G6P, although this inhibition is not released by the presence of inorganic phosphate.
Not a lot is known about the functions of HKIII. It may be an inactive gene duplication or remnant. Under basal conditions, it is not expressed to appreciable levels in any major tissues, and studies on its biological activity it is inhibited by glucose at physiological concentrations. However, some studies suggest that it may be expressed during cellular stress responses, such as hypoxia, although its function in these types of responses is not currently understood.
Hexokinase IV or Glucokinase is specifically expressed within the liver and pancreas. HKIV is cytoplasmic and not tethered to the mitochondria. Activity within the pancreas serves as a sensor for the release of insulin, and in the liver for the production of G6P that will fuel glycogen production. HKIV has a higher Km than HKI and HKII, thus it does not work efficiently at low concentrations of glucose. However, it is NOT inhibited by the product, G6P. Thus, it will continue to make G6P, even when levels are high. This helps to explain the high levels of glycogen that are stored within liver tissue, but not elsewhere in the body. This also ensures that the sensor system in the pancreas will accurately read blood glucose levels.
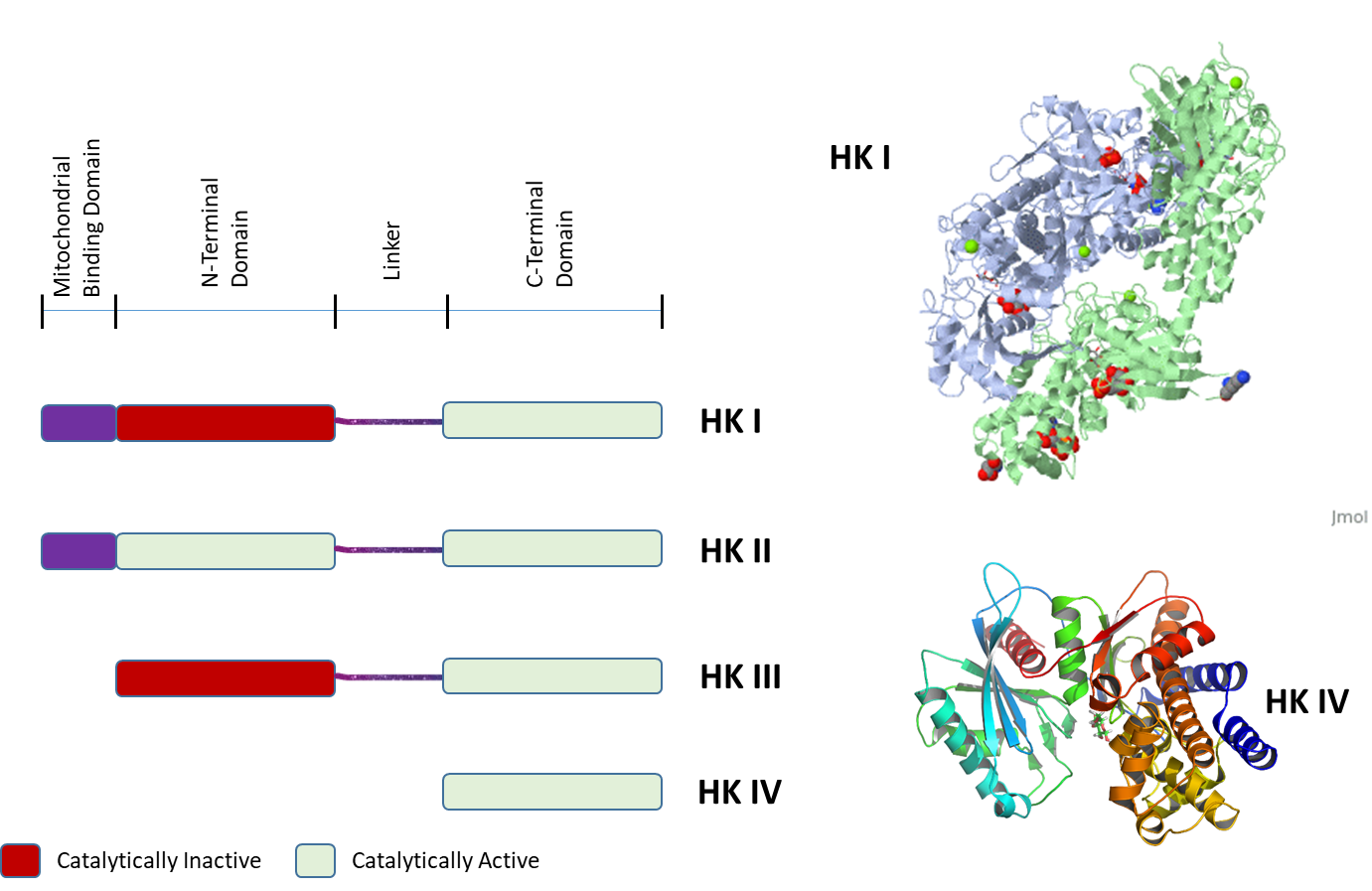
The four isozymes of HK share high homology with one another and appear to have arisen from gene duplication events, as shown in Figure \(\PageIndex{4}\). The left-hand panel of Figure 15.5.4 shows the linear protein domains of the different HK isozymes. Both HKI and HKII contain an N-terminal domain that localizes the protein to the mitochondrial membrane. HKI, II, and III all contain two repeating catalytic domains in the N- and C-terminals. However, mutations in the N-terminal domain in HKI and HKIII render them inactive. Both catalytic domains in HKII retain activity. HKIV (Glucokinase) is the most truncated isozyme, only containing the C-terminal catalytic domain. The lower right diagram shows the ribbon diagram of HKIV. Upstream promoter regions of HKIV (not shown in this diagram) also differ, allowing for controlled expression in the liver and pancreas. Feedback inhibition in HKI and HKII occurs through the N-terminal catalytic domain. The upper diagram on the right shows an HKI dimer complex with an ATP analog, glucose, G6P, and Mg2+ ion. HKI dimerizes when concentrations of the inhibitor, G6P, are high enough. Dimerization reduces the biological activity of the enzyme in brain tissue. Dimerization of HKI can be reversed in the presence of low levels of inorganic phosphate.
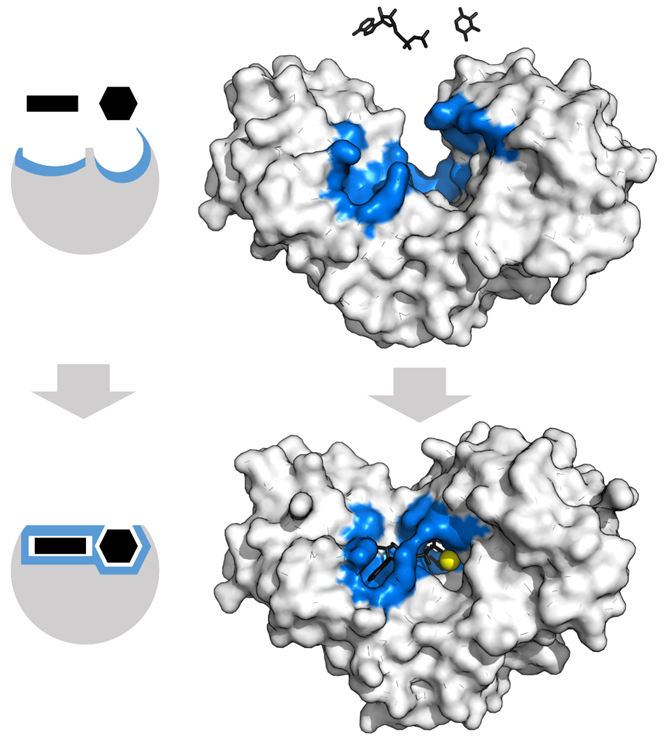
HKIV is also a good model for understanding enzyme conformation change during the reaction. HKs change shape by induced fit upon substrate binding. HKIV has a large induced fit motion that closes over the substrates ATP and xylose, as shown in Figure \(\PageIndex{5}\). The binding sites are shown in blue, substrates in black, and the Mg2+ cofactor in yellow (PDBs:2E2N,2E2Q).
In summary, the isozyme expression patterns of HKs differentially regulate the fate of glucose within those tissues. Within brain tissue and red blood cells where only HKI is present, glucose is predominantly used in the glycolytic pathway for energy production. In muscle tissue, the presence of HKII allows for increased use of glucose for the formation of glycogen. HKIV expression in the pancreas and liver allows for the homeostatic regulation of blood glucose levels and stockpiling of glucose in the form of glycogen.
Phosphofructokinase-1 Regulation
Recall that phosphofructokinase-1 (PFK1) mediates the third step in the glycolytic pathway with the conversion of fructose 6-phosphate to fructose 1,6-bisphosphate, as shown in Figure \(\PageIndex{6}\). The PFK1 reaction is the first irreversible reaction of glycolysis. It also represents the committed step within the pathway. The phosphorylation of fructose-6-phosphate (F6P) to fructose-1,6-bisphosphate (F1,6BP) commits the F1,6BP to continue through the glycolytic pathway. It cannot be utilized for any other purpose at that point. F6P, on the other hand, could be converted back into glucose-6-phosphate and used for many different purposes (ie glycogen synthesis, nucleotide synthesis, or hexosamine synthesis). Because of the committed nature of this step, PFK1 is one of the most important control points in the glycolytic pathway.
The PFK1 enzyme is composed of a tetramer that can contain different combinations of three types of subunits: muscle (M), liver (L), and platelet (P). The composition of the PFK1 tetramer differs according to the tissue type it is present in. For example, mature muscle expresses only the M isozyme, therefore, the muscle PFK1 is composed solely of homotetramers of M4. The liver and kidneys express predominantly the L isoform. In erythrocytes, both M and L subunits randomly tetramerize to form M4, L4, and the three hybrid forms of the enzyme (ML3, M2L2, M3L). As a result, the kinetic and regulatory properties of the various isoenzymes pools are dependent on subunit composition. Tissue-specific changes in PFK1 activity and isoenzymes content contribute significantly to the diversities of glycolytic and gluconeogenic rates which have been observed for different tissues
PFK1 is an allosteric enzyme and has a structure similar to that of hemoglobin in so far as it is a dimer of a dimer, as shown in Figure \(\PageIndex{7}\). One half of each dimer contains the ATP binding site, whereas the other half the substrate (fructose-6-phosphate or (F6P)) binding site, as well as a separate allosteric binding site, that can bind with ADP or AMP. All isoforms of PFK1 are activated by the allosteric binding of ADP or AMP. This indicates a low energy state within the cell and the need for glycolysis and energy generation. Allosteric inhibitors include high levels of ATP and Citrate. Note that ATP is a substrate of this enzyme and has the normal substrate binding site. When there is enough ATP present that it can also bind allosterically to the enzyme, it will act as an inhibitor. Citrate, the first molecule in the Kreb's Cycle (Citric Acid Cycle), can also act as an allosteric inhibitor of PFK-1. High levels of citrate that no more pyruvate is needed to generate ATP through oxidative phosphorylation. We are also going to see that Fructose 2,6-bisphosphate is predominantly a regulator of PFK1 in Liver Cells, where it serves as an activator.
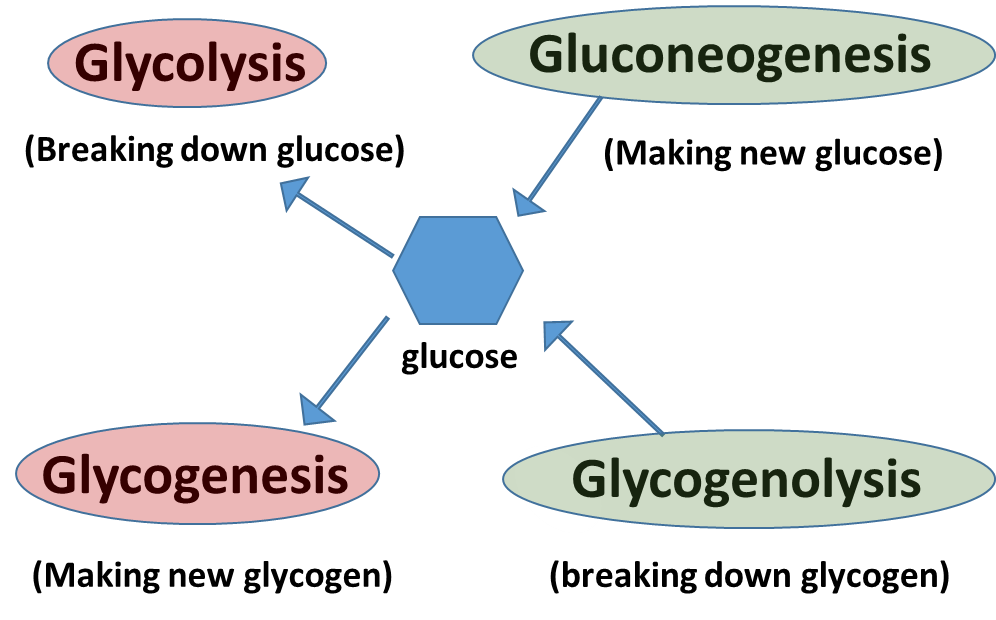
Before we discuss the formation and use of Fructose 2,6-bisphosphate and its role in the regulation of PFK1, let’s review opposing glucose utilization/production pathways within the liver, as shown in Figure \(\PageIndex{8}\). Two opposing pathways within the liver are glycolysis (the breakdown of glucose) and gluconeogenesis (the formation of glucose). It would be unproductive to have both of these pathways operating at the same time. Thus, if one pathway is needed, then the other one needs to be turned off. We previously saw this same type of control for glycogenesis (the formation of glycogen) and glycogenolysis (the breakdown of glycogen). When one of these pathways is upregulated, the other needs to be downregulated.
In the resting state of the liver, when blood glucose levels are in the homeostatic range, gluconeogenesis will be shut off, as it is very expensive to make glucose and the liver will only invest in making glucose if blood glucose levels fall critically low (ie extreme exercise or long term fasting). In the resting state, the liver will use the glycolytic pathways to supply normal levels of ATP energy for the maintenance of housekeeping processes. Glycogenesis and glycogenolysis will be in balance or equilibrium as needed to augment the supply of glucose entering the system from the bloodstream.
If glucose in the blood drops below homeostatic levels, the pancreas will release glucagon and begin this hormone-signaling pathway that causes the liver to release glucose into the bloodstream. Thus, glucagon signaling leads to the downregulation of glycolysis and glycogenesis, so it can shunt glucose pools to the bloodstream. It also leads to an increase in glycogenolysis or the breakdown of glycogen. During this time, liver cells are predominantly generating ATP from lipids, rather than carbohydrates. Thus, glycolysis can be inhibited to promote the release of glucose into the bloodstream.
If cellular demand for glucose is high, liver cells will also turn on the gluconeogenesis pathway and make glucose new from non-carbohydrate precursors (which it then exports into the bloodstream for delivery to tissues). This is an energy-intensive pathway. The major site of gluconeogenesis is the liver, with a small amount also taking place in the kidney. Little gluconeogenesis takes place in the brain, skeletal muscle, or heart muscle. It just does not make energetic sense to do that! It costs more energy for cells to make glucose than they can get from breaking it down in oxidative phosphorylation. This cost can be dealt with by doing it in the liver and then releasing it into the bloodstream to fuel activities in the brain, heart, and skeletal muscles.
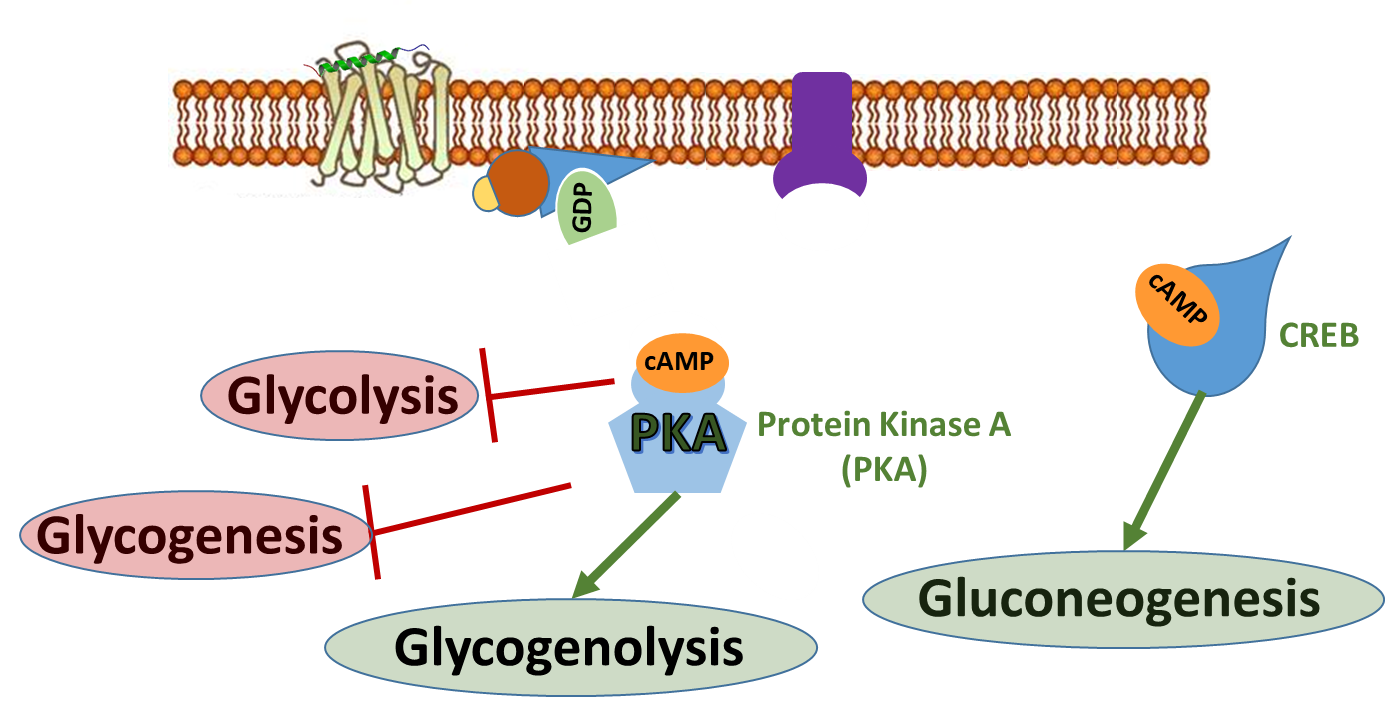
To do this, glucagon stimulates that lovely signaling pathway that you are all now familiar with (reviewed in Figure \(\PageIndex{9}\))! Within the liver, it activates the G-protein coupled receptor which in turn activates the downstream G-protein. Adenylate Cyclase is activated and produces the second messenger, cAMP. cAMP binds with the CREB protein and activates the transcription of proteins involved in gluconeogenesis. The cAMP also binds with Protein Kinase A and upregulates the activity of glycogen phosphorylase resulting in the breakdown of glycogen. It also down-regulates the activity of glycogen synthase to inhibit glycogenesis. In this section, we will also see how PKA activation will down-regulate the activity of the glycolytic pathway as well.
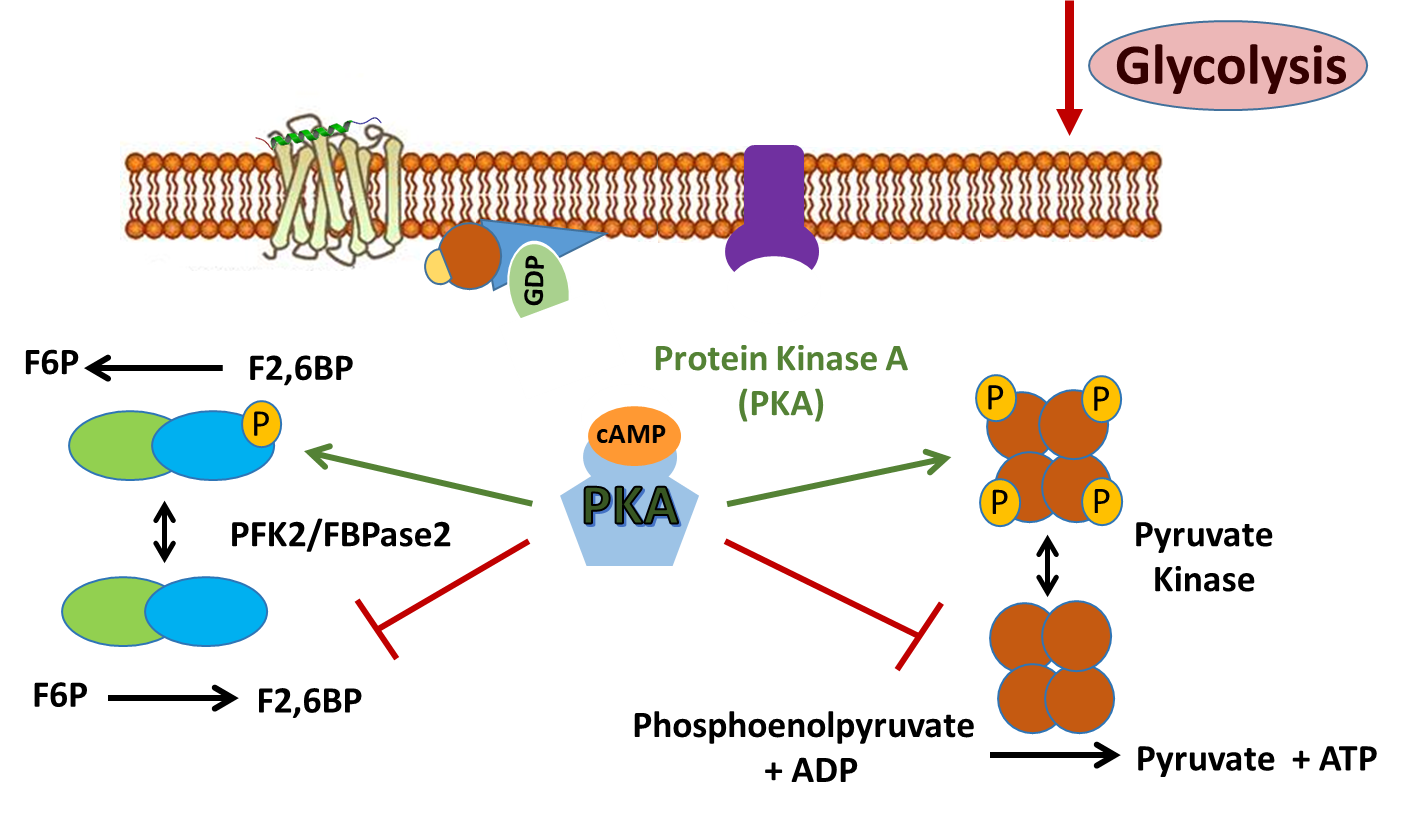
Regulation of the glycolytic pathway in response to this signaling occurs through the regulation of the PFK-2/FBPase-2 Enzyme. The activity of this enzyme is controlled through the PKA signaling cascade. This enzyme is responsible for phosphorylating fructose 6-phosphate to the fructose 2,6-bisphosphate form. Note that this bisphosphate form of fructose is DIFFERENT than the bisphosphate form utilized in the glycolytic pathway. The glycolytic pathway requires fructose 1,6-bisphosphate that is formed from the PFK1 enzyme (or Step 3 of the glycolytic pathway). The PFK-2/FBPase-2 is a separate enzyme altogether and not involved directly in the glycolytic pathway. However, we noted previously that fructose 2,6-bisphosphate can serve as an allosteric activator of the PFK1 enzyme.
The PFK-2/FBPase-2 Enzyme is a dual-purpose enzyme, as shown in Figure \(\PageIndex{10}\). Half of the protein has kinase activity and can phosphorylate fructose-6-phosphate to fructose-2,6-bisphosphate. The other half of the enzyme contains a phosphatase that can cleave off the phosphate group from the 2-position and restore fructose-6-phosphate. Note that both activities are not functional at the same time! The enzyme activity is determined by the phosphorylation state. When the protein is dephosphorylated, the PFK-2 enzyme is active, leading to the production of fructose-2,6-bisphosphate. This molecule can then bind with PFK-1 in the glycolytic pathway and increase its activity. If the protein is phosphorylated by PKA during glucagon signaling, the FBPase is activated and the kinase activity is inhibited. This leads to the dephosphorylation of fructose at the 2-position and the release of fructose-6-phosphate.
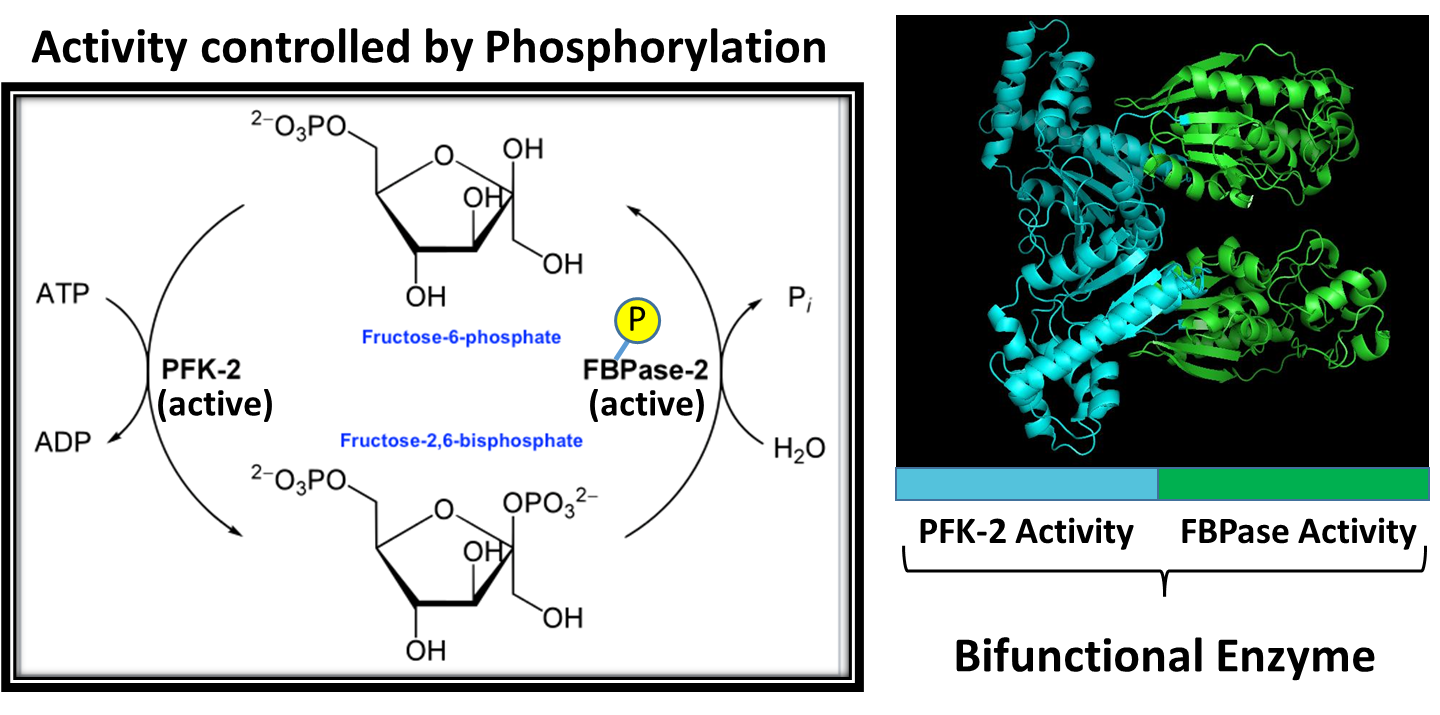
As shown in Figure 15.5.10, the PFK-2/FBPase-2 Enzyme is responsible for phosphorylating fructose 6-phosphate to the fructose 2,6 bisphosphate form. Note again that this bisphosphate form of fructose is DIFFERENT than the bisphosphate form utilized in the glycolytic pathway. The glycolytic pathway requires fructose-1,6-bisphosphate that is formed from the PFK1 enzyme (or Step 3 of the glycolytic pathway). If there is a lot of fructose-6-phosphate around (ie you just drank high fructose corn syrup in your sugary energy drink) it can support both the formation of fructose 1,6-bisphosphate by PFK1 and the production of fructose 2,6-bisphosphate by PFK-2. Fructose 2,6-bisphosphate will then bind with PFK1 and increase its activity converting fructose 6-phosphate into fructose-1,6-bisphosphate, as shown in Figure \(\PageIndex{11}\).
However, during glucagon signaling, you need to shut down this fast-track upregulation of PFK1 by fructose-2,6-bisphosphate and turn down the glycolytic pathway. To do this, Protein Kinase A will phosphorylate the PFK-2/FBPase-2 enzyme and alter its activity. The kinase activity is inhibited and the phosphorylase activity is turned on. Figure \(\PageIndex{12}\) provides a summary of this pathway control. In the presence of glucagon, PKA will phosphorylate the PFK-2/FBPase-2 enzyme causing the kinase activity to be switched off and the phosphatase activity to be switched on. Dephosphorylation of fructose-2,6-bisphosphate recovers fructose-6-phosphate (F6P). F6P can then go through the reverse isomerase reaction and recover glucose-6-phosphate (G6P). G6P will then be transported to the rER where it will be dephosphorylated and then free glucose can be released back into the blood.
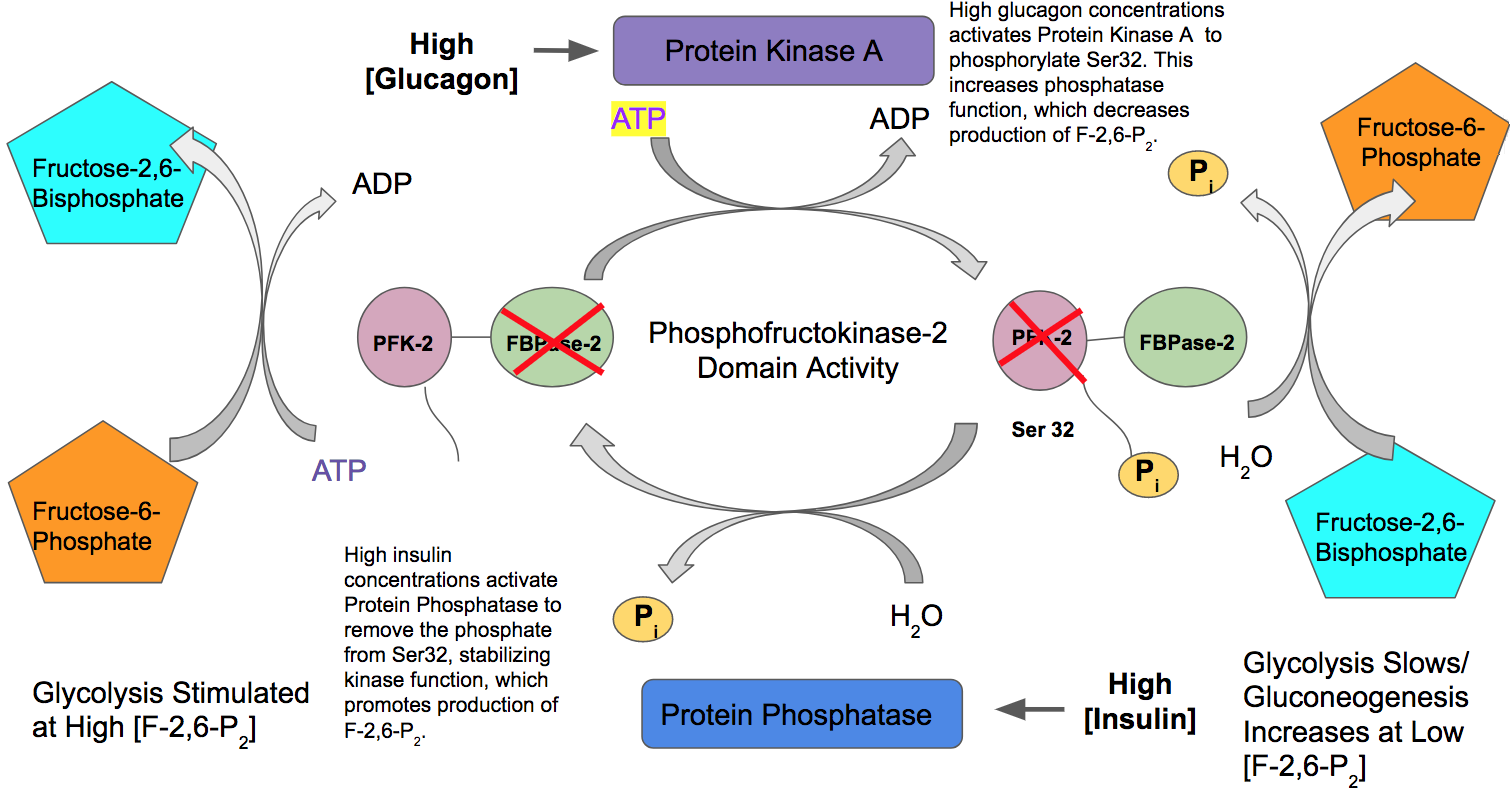
The opposite holds for insulin signaling. High insulin concentrations result in the activation of protein phosphatase and the dephosphorylation of the PFK-2/FBPase-2 enzyme. In the dephosphorylated state, PFK-2 activity is high and the FBPase-2 activity is low, which will stimulate PFK1 and the glycolytic pathway.
Pyruvate Kinase
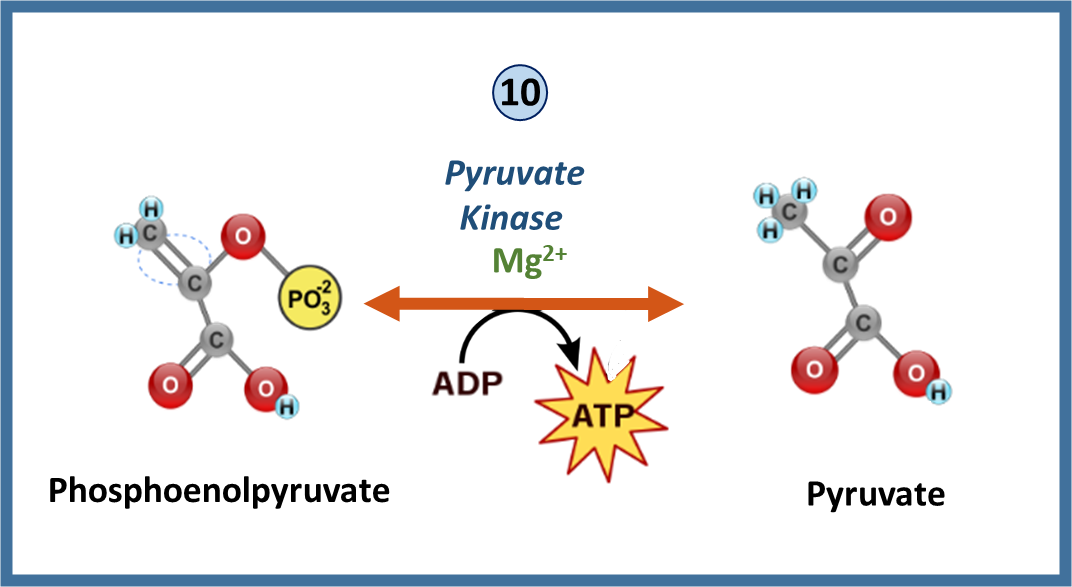
Recall that pyruvate kinase mediates the final reaction during glycolysis resulting in the production of pyruvate and ATP as shown in Figure \(\PageIndex{13}\). Similar to PFK1 this enzyme is also a key regulatory component within the pathway.
The pyruvate kinase enzyme exists as a tetramer, that is built from the combination of different isozymes expressed in different tissues. There are three major isozymes of pyruvate kinase, the L form that is predominantly found in the liver, the R form that is predominantly found in erythrocytes, and the M1 form in muscle and brain, and the M2 form that is expressed in fetal tissue and at some level in most adult tissues. The L and R forms are splice variants that arise from the same gene locus, and the M1 and M2 forms are also splice variants that arise from the same gene locus.
We will focus on some of the general regulatory mechanisms common to most of the isozymes of pyruvate kinase, starting with the activator, fructose 1,6-bisphosphate (FBP). Because FBP is an earlier product within the same metabolic cascade, the activation of pyruvate kinase enzymes by FBP is known as feedforward stimulation. All of the isozymes, except for the M1 form are stimulated by the binding of FBP to the enzyme. Similarly, all of the pyruvate kinase isozymes are inhibited by the product of the reaction, ATP (or high energy load), and high levels of alanine. Alanine can be converted to pyruvate in one enzymatic step. Thus, pyruvate serves as a metabolic intermediate in the formation of alanine. If high levels of alanine are present, this indicates that there is a high energy load within the cell (ie that the cell is full of building blocks to make new macromolecules and is not in the need of more energy). Thus, high levels of alanine serve as a negative regulator of the pyruvate kinase family of enzymes.
The liver isozyme of pyruvate kinase is also regulated through protein phosphorylation, as shown in Figure \(\PageIndex{14}\). Similar to the PFK-2 activity of the PFK-2/FBPase-2 enzyme, the liver isozyme of Pyruvate Kinase is also downregulated during glucagon signaling. Protein kinase A phosphorylates Pyruvate Kinase inhibiting its activity and preventing the conversion of phosphoenolpyruvate to pyruvate. Dual regulation of the glycolytic pathway during glucagon signaling helps to ensure that glucose resources will be diverted away from cellular use by the liver and released into the blood stream to restore homeostatic blood glucose levels.
Fructose Regulatory Bypass
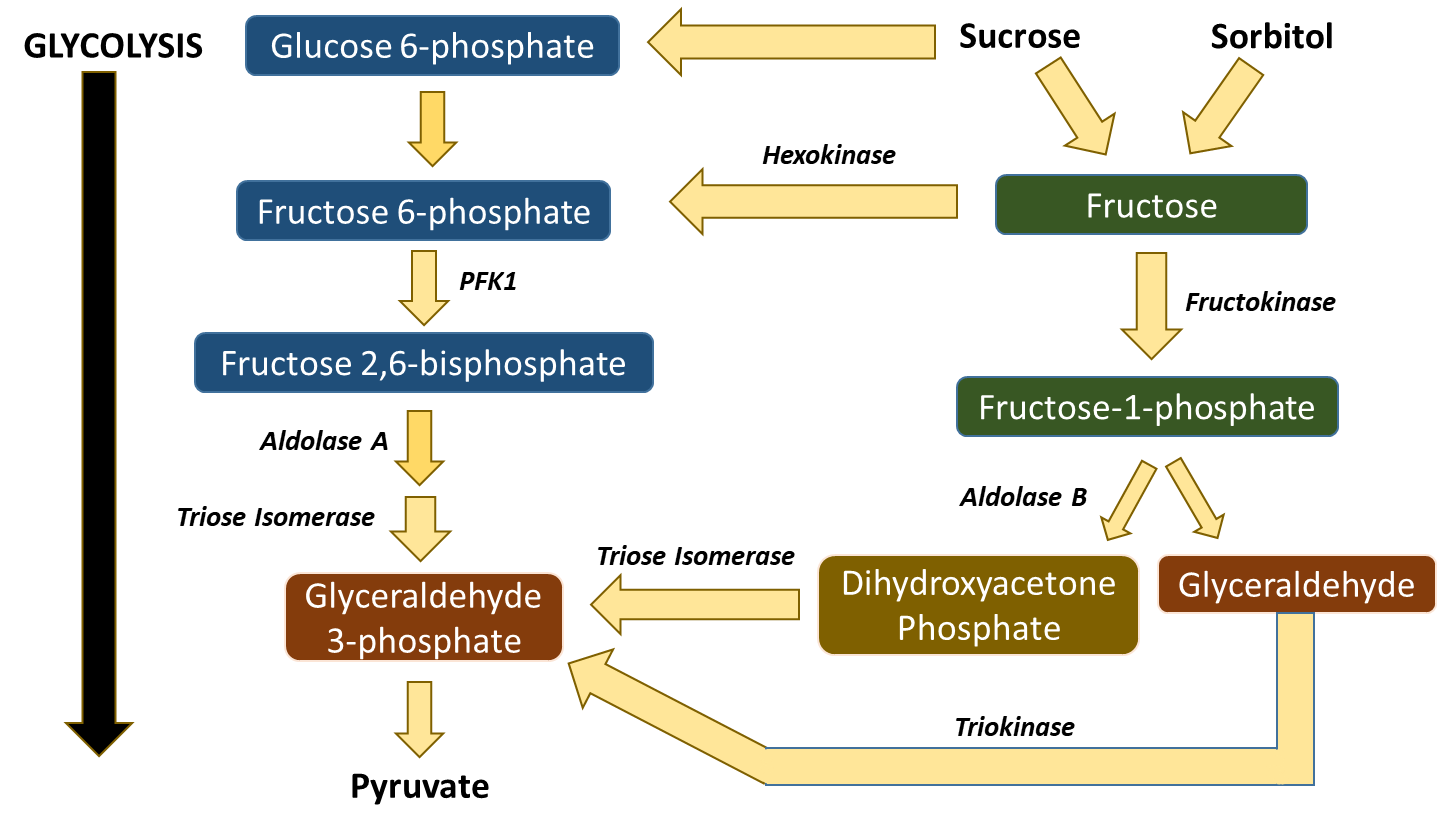
Other sugars from the diet can also enter into the glycolytic pathway, as shown in Figure \(\PageIndex{15}\). Galactose is converted in a four-step process to Glucose-6-phosphate and mannose can be converted to fructose-6-phosphate. Within most of the body’s tissues, fructose can also be converted into fructose-6-phosphate by hexokinase. However, in the liver and kidneys, there is an alternative route that fructose from the diet can take to enter into the glycolytic pathway. This pathway is concerning because it bypasses two of the major regulatory steps of the glycolytic pathway, the hexokinase step and the PFK1 step. Within the liver and kidneys, fructose can also be converted into fructose-1-phosphate by the enzyme fructokinase. The other isozyme of Aldolase, Aldolase B, can cleave the fructose-1-phosphate into 2 three carbon units, dihydroxyacetone phosphate, and glyceraldehyde. Dihydroxyacetone phosphate can be converted to glyceraldehyde 3-phosphate by Triose Isomerase and then continue into the glycolytic cascade. Glyceraldehyde can be phosphorylated to Glyceraldehyde 3-phosphate by Triokinase. This is an unregulated system that can flood the Kreb Cycle with high levels of pyruvate if high levels of fructose enter the cell (i.e. from high fructose corn syrup, sucrose, and other sweeteners common to the Westernized diet). The excess pyruvate can then be shunted into fatty acid biosynthesis for long-term storage in the form of triglycerides. If the pathway is overutilized by consuming too much sucrose and high fructose corn syrup, this can lead to the development of Hypertriglyceridemia (or the heightened increase of body fat). With this, we will end our discussions of glycolysis. In the next section, we will look at the complementary and opposite pathway, gluconeogenesis.