6: Acid-Fast, Spores, and Capsule Stains
- Page ID
- 105487
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- List the steps necessary for successfully performing an acid-fast stain.
- Explain how each of the steps contribute to the final outcome in an acid-fast positive vs. negative microorganism.
- Identify organisms as either acid-fast positive or negative based on staining.
- Identify the function of endospores, capsule, and flagella.
- Characterize microbes based on the presence of endospores, capsule, and flagella.
- Determine the necessary requirements for staining these structures.
- Identify these structures in microscopic images.
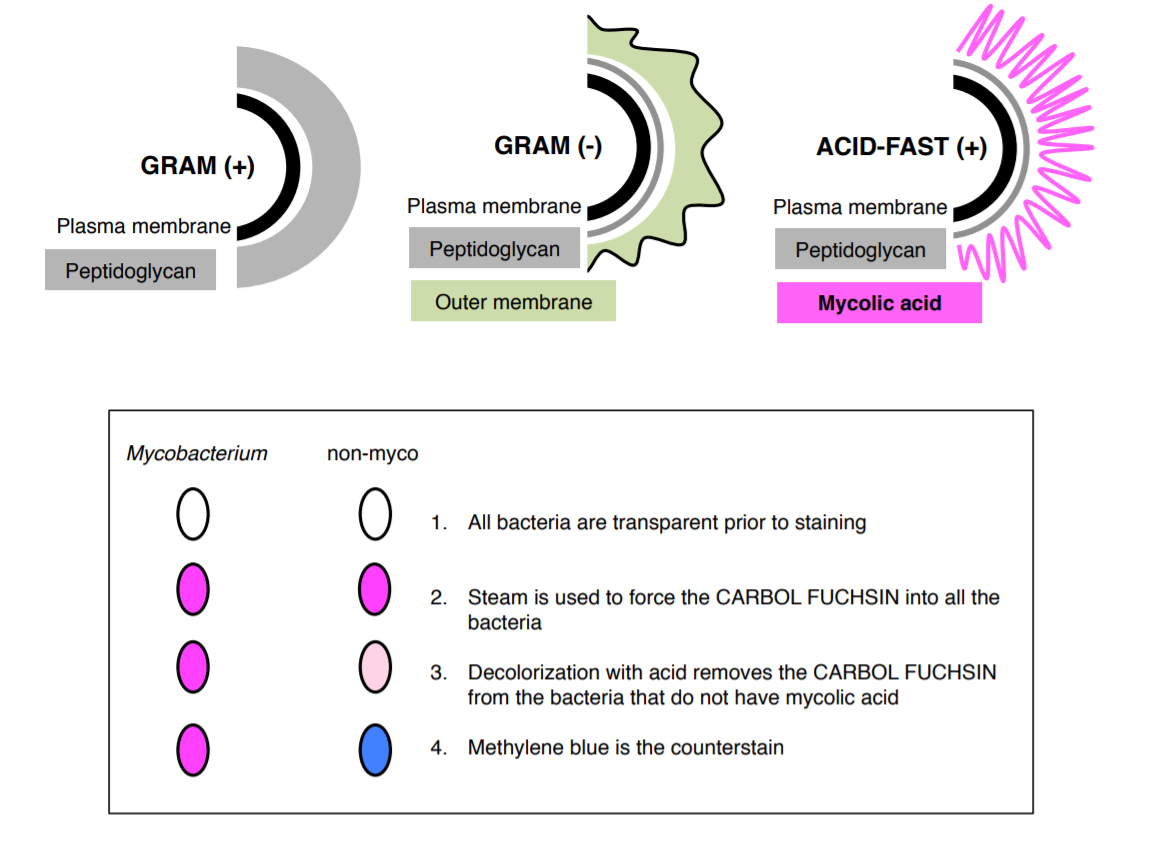
ACID-FAST
Acid-fast stain is a differential stain used to identify acid-fast organisms such as members of the genus Mycobacterium. Acid-fast organisms are characterized by wax-like, nearly impermeable cell walls; they contain mycolic acid and large amounts of fatty acids, waxes, and complex lipids. This type of cell wall is resistant to most compounds, therefore acid-fast organisms require a special staining technique.
The primary stain used in acid-fast staining, carbol fuchsin, is lipid-soluble and contains phenol, which helps the stain penetrate the cell wall. This is further assisted by the addition of heat in the form of heat (steam). Steam helps to loosen up the waxy layer and promotes entry of the primary stain inside the cell. The smear is then rinsed with a very strong decolorizer, which strips the stain from all non-acid-fast cells but does not permeate the cell wall of acid-fast organisms. The decolorized non-acid-fast cells then take up the counterstain, which in our case is methylene blue.
1. Working in pairs, prepare THREE slides as directed below (2 for back-up and one to stain).
2. Prepare an emulsion on each slide with 4 loopfuls of Staphylococcus epidermidis from your broth culture onto the slide.
3. Then, add one loopful of Mycobacterium chelonae (these are your acid fast positive bacteria) and mix the two bacteria together.
4. Allow the slide(s) to air dry on the slide warmers (while these slides are drying, prepare your slides for the spore stain).
5. Once the liquid has completely evaporated, heat fix the bacteria by passing it through your flame three times.
6. Make sure the slide rack on top of your beaker is completely level. Then, bring your water to boil while the slides are drying.
You only need about 200 milliliters of water. If you add more, you will be waiting all lab period for your water to boil.
7. Once the water is boiling, place your slide on the slide rack above the boiling water.
8. Cover the area of your smear on the slide with a square piece of PRECUT paper towel. Make sure none of the paper is hanging off the slide.
9. Carefully apply the CARBOL FUCHSIN stain to the paper towel.
If a stain appears in the water you are boiling, please stop and discard the stained water in the liquid waste disposal. The fumes from carbol fuchsin can be toxic.
10. Steam with the stain on the slide for 7 minutes while continuously applying more stain so the paper square never dries out.
11. Gently remove the paper with forceps and discard it in the small waste paper cup that will be provided on your bench. Then, rinse the slide with water.
12. Put the slide on your staining basin and gently rinse with water.
13. Decolorize with 6 drops of acid alcohol (not ethanol from your gram stain kit), then rinse with water.
14. Counterstain with methylene blue for 2 minutes.
15. Rinse with water and blot dry with bibulous paper (do not use the slide warmer).
16. Examine under the 100X objective lens with oil immersion and record your results.
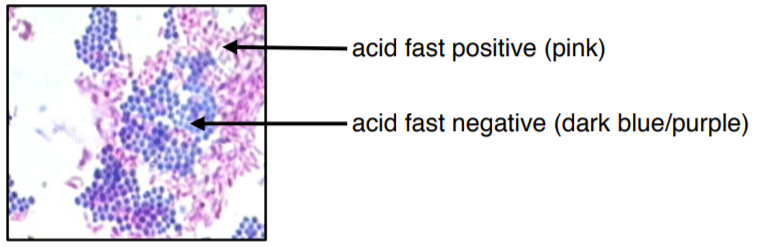
The colors of this image may be slightly off due to printing/copying.
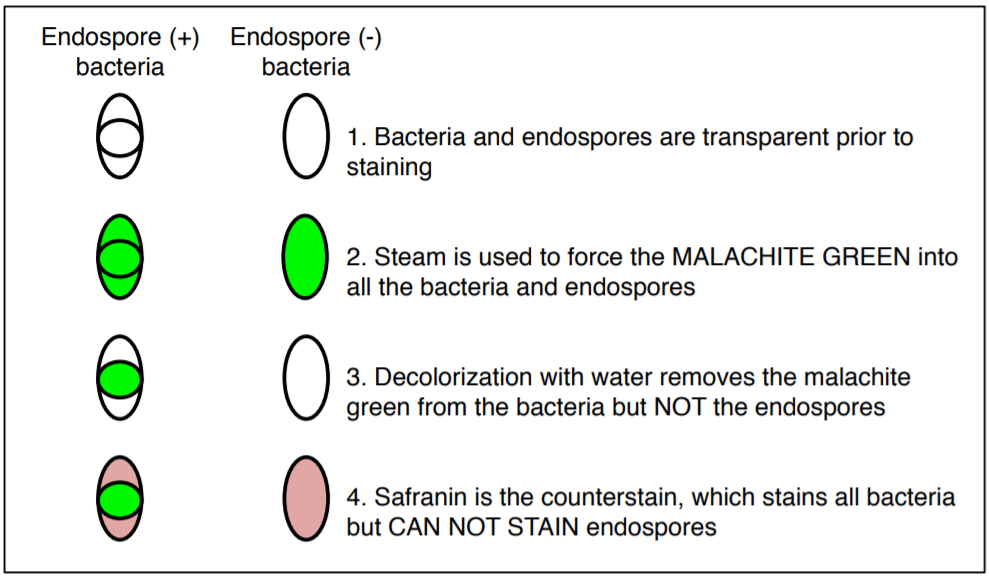
SPORE STAIN
The endospore stain is a differential stain used to visualize bacterial endospores. An endospore is a dormant form of a bacterium, which some species of bacteria produce under stressful conditions such as poor nutrition, high temperatures, or dry environments. The outer layer is composed of keratin which resists staining. The malachite-green stain is forced into the spore using steam. Spores can be central, terminal, and subterminal. This stain is commonly used to detect spores produced from the genera of Bacillus and Clostridium.
1. Prepare two slides with emulsions one from plate A and one from plate B.
2. Heat fix the slides and place them on the slide rack above the boiling water.
3. Cover the area of your smear on each slide with a square piece of PRECUT paper towel.
4. Carefully apply the MALACHITE GREEN stain to the paper towel.
5. Steam for 10 minutes and keep the paper soaked with the stain during this time.
6. Gently remove the paper with forceps, discard in the small waste paper cup that will be provided on your bench, and then rinse the slide with water.
7. Put the slides on your staining basin and gently rinse with water.
8. Counterstain with SAFRANIN for 1 minute and then rinse with water. Blot dry with bibulous paper.
9. Examine under the 100X objective lens with oil immersion and record your results.
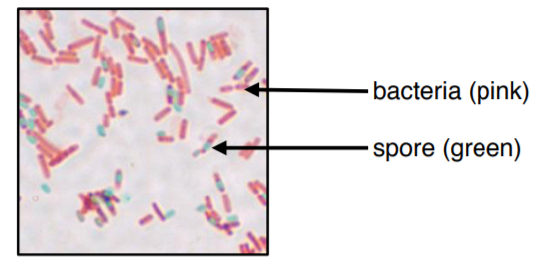
The colors of this image may be slightly off due to printing/copying.
CAPSULE STAIN
Most capsules are composed of polysaccharides or polypeptides which are a thick, detectable, and discrete layer outside the cell wall. Some capsules have well-defined boundaries while some have fuzzy, trailing edges. Capsules protect bacteria from the phagocytic action of immune cells and allow pathogens to invade the body. If a pathogen loses its ability to form capsules, it often ceases to be pathogenic.
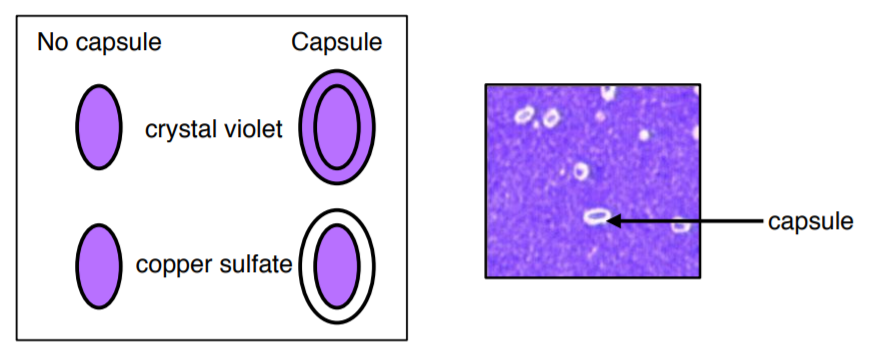
1. Label and prepare a slide with an emulsion of Klebsiella pneumoniae.
2. Let it air dry (DO NOT USE THE SLIDE WARMER, DO NOT HEAT FIX).
3. Stain with 1% crystal violet for 2 minutes (DO NOT USE CRYSTAL VIOLET FROM GRAM STAIN).
4. VERY GENTLY rinse with 6 drops of copper sulfate.
5. Let it air dry on the counter (do not use slide warmer).
6. Examine under the 100X objective lens with oil immersion and record your results.

