15.3: DNA sequencing
- Page ID
- 88995
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)For some perspective, we should note that RNA sequencing came first, when Robert Holley sequenced a yeast transfer RNA (tRNA) in 1965. The direct sequencing of tRNAs was possible because they are small, short nucleic acids, and because many of the bases in tRNAs are chemically modified after transcription, making them easy to identify.
Twelve years later, in 1977, two different methods for sequencing DNA were reported. One method, developed by Allan Maxam and Walter Gilbert, involved fragmenting DNA, sequencing the small fragments, and then aligning the overlapping sequences of the short fragments to assemble longer sequences. This became known as Maxam-Gilbert DNA sequencing. The other method was developed by Frederick Sanger and colleagues in England: the DNA synthesis-based dideoxy-DNA sequencing technique. Sanger and Gilbert shared a Nobel Prize in Chemistry in 1983 for their DNA-sequencing work. However, because of its simplicity, Sanger’s dideoxy method quickly became the standard for sequencing all manner of cloned DNAs, including the first complete sequencing of a genome—that of a bacterial virus called bacteriophage φX174.
Early improvements in recombinant DNA technology were happening at the same time as advances in sequencing. More efficient, rapid cloning and sequencing of DNA from increasingly diverse sources led to genome projects. The first of these were on genomes of important model organisms—for example, E. coli, C. elegans, S. cerevisiae (yeast), and of course, us! By 1995 Craig Venter and colleagues at the Institute for Genomic Research completed an entire bacterial genome sequence (of Haemophilus influenzae); by 2001 Venter’s private group, along with Francis Collins and colleagues at the National Institutes of Health, had published a first draft of the sequence of the human genome. Venter had proven the efficacy of a whole-genome-sequencing approach called shotgun sequencing, which was much faster than the gene-by-gene, fragment-by-fragment, “linear” sequencing strategy being used by other investigators (more on shotgun sequencing later!). Since Sanger’s dideoxynucleotide DNA-sequencing method remains a common, economical methodology, let’s consider the basics of the protocol.
15.3.1 Manual DNA Sequencing
Given a template DNA (e.g., a plasmid cDNA), Sanger used in vitro replication protocols to demonstrate that he could do the following: • Replicate DNA under conditions that r
- Replicate DNA under conditions that randomly stopped nucleotide addition at every possible position in growing strands •
- Separate and then detect these DNA fragments of replicated DNA
Recall that DNA polymerases catalyze the formation of phosphodiester linkages by linking the \(\alpha\) phosphate of a nucleotide triphosphate to the free 3’ OH of a deoxynucleotide at the end of a growing DNA strand. Recall also that the ribose sugar in the deoxynucleotide precursors of replication lack a 2’ OH (hydroxyl) group. Sanger’s trick was to add dideoxynucleotide triphosphates to his in vitro replication mix. The ribose on a dideoxynucleotide triphosphate (ddNTP) lacks a 3’ OH, as well a 2’ OH (Figure 15.14).

Adding dideoxynucleotides to a growing DNA strand stops replication. Lacking an OH at the 3’ end of the strand, DNA polymerase can’t catalyze a dehydration synthesis to form the next phosphodiester linkage. Because they can stop replication in actively growing cells, ddNTPs such as dideoxyadenosine (tradename, cordycepin,) are anticancer chemotherapeutic drugs.
266 Treating Cancer with Dideoxynucleosides
A look at a manual DNA sequencing reveals what is going on in the sequencing reactions. Four reaction tubes are set up, each containing the template DNA to be sequenced. Each tube also contains a primer of known sequence and the required deoxynucleotide precursors necessary for replication. Figure 15.15 (below) shows the setup for manual DNA sequencing.

A different ddNTP, (ddATP, ddCTP, ddGTP, or ddTTP) is added to each of the four tubes. Then, DNA polymerase is added to each tube to start the DNA-synthesis reaction. During DNA synthesis, different-length fragments of new DNA accumulate as ddNTPs are incorporated at random, opposite complementary bases in the template DNA being sequenced. A short time after adding the DNA polymerase, the mixture is heated to separate the DNA strands, and fresh DNA polymerase is added to replace the enzyme destroyed by heating and to repeat the synthesis reactions. These reactions are repeated as many as thirty times in order to produce enough radioactive DNA fragments to be detected. Expectations for these sequencing reactions in the four tubes are illustrated in Figure 15.16.
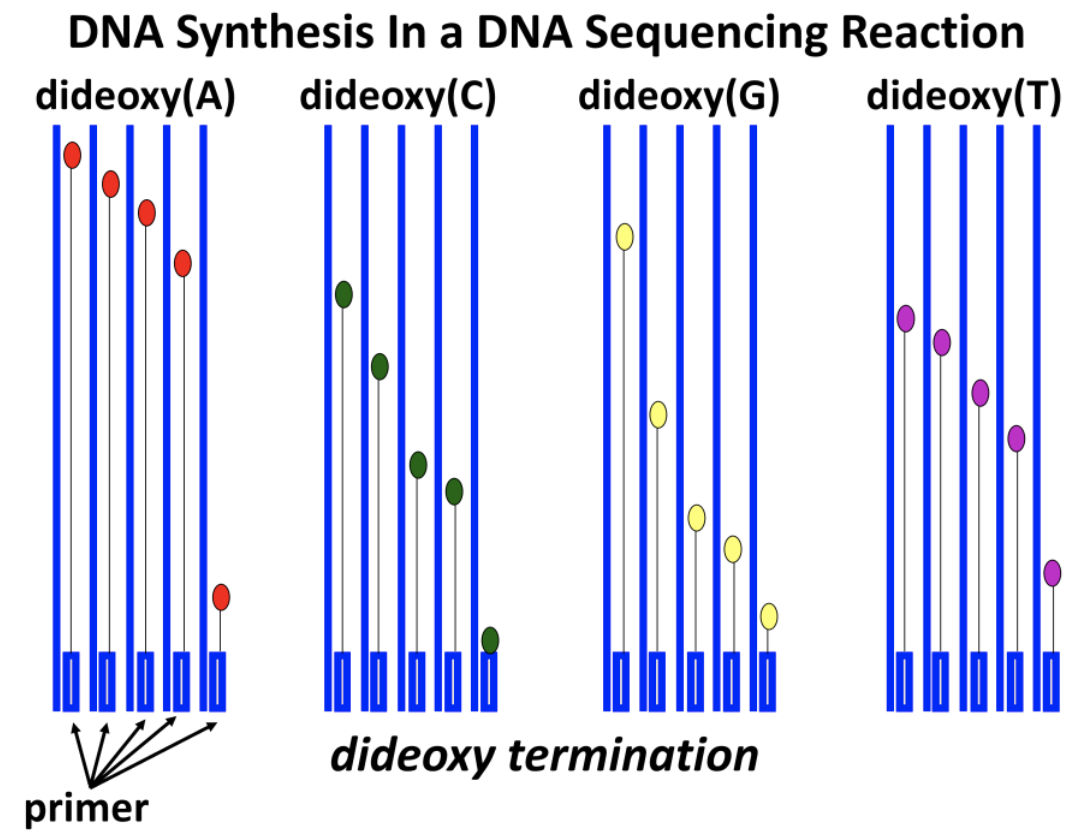
dNTP and ddNTP concentrations are carefully controlled in DNA sequencing reactions. What might result if ddNTP levels are too high?
When the Taq DNA polymerase from the thermophilic bacterium Thermus aquaticus became available (more later!), it was no longer necessary to add fresh DNA polymerase after each replication cycle, because the heating step required to denature new DNA made in each cycle would not destroy the heat stable Taq DNA polymerase.
Thanks to Taq polymerase, the many heating and cooling cycles required for what became known as dideoxy-chain-termination DNA sequencing were soon automated using inexpensive programmable thermocyclers. Since a small amount of a radioactive deoxynucleotide (usually \({}^{32}\)P-labeled ATP) was present in each reaction tube, all newly made DNA fragments are radioactive. After electrophoresis to separate the new randomly terminated DNA fragments in each tube, autoradiography of the electrophoretic gel reveals the position of each terminated fragment. The DNA sequence can then be read from the gel as illustrated in the simulated autoradiograph (Figure 15.17).

The DNA sequence can be read by reading the bases from the bottom of the film, starting with the C at the bottom of the C lane. Try reading the sequence yourself!
15.3.2 Automated First-Generation DNA Sequencing
The first semi-automated DNA sequencing method was invented in Leroy Hood’s California lab in 1986. Though the method still used Sanger’s dideoxy sequencing, a radioactive phosphate-labeled nucleotide was no longer necessary. Instead, each dideoxynucleotide (ddNTP) in a sequencing reaction is tagged for detection with a different fluorescent dye.
Sequence reaction products are electrophoresed on an automated DNA sequencer. The migrating dideoxy-terminated DNA fragments pass through a beam of UV light, and a detector “sees” the fluorescence of each DNA fragment. The color and order of the fragments is sent to a computer, which generates a colored plot showing the length and order of the fragments, and thus their sequences (Figure 15.18).

A most useful feature of this sequencing method is that a template DNA could be sequenced in a single tube containing all the required enzymatic components, as well as all four of the dideoxynucleotides! That’s because the fluorescence detector in the sequencing machine separately sees all the short ddNTP-terminated fragments as they move through the electrophoretic gel. Hood’s innovations were quickly commercialized, making major sequencing projects possible (including many genome projects). Automated DNA sequencing rapidly augmented large sequence databases in the United States and Europe. In the United States, the National Center for Biological Information (NCBI) maintains a sequence database and archives virtually all DNA sequences determined worldwide. New “tiny” DNA sequencers have made the requirements for sequencing DNA so portable that in 2016, one was even used in the International Space Station (see the photo at the top of this chapter!). New tools and protocols (some described in this chapter) are used to find, compare, and globally analyze DNA sequences almost as soon as they get into the databases.
268 Automated Sequencing Leads to Large Genome Projects
What chemical properties of Taq polymerase could explain the stability of this enzyme at temperatures near \(100^{\circ}C\)?
15.3.3 Shotgun Sequencing
Large-scale sequencing targets entire prokaryotic and (typically much larger) eukaryotic genomes. The latter require strategies that either sequence long DNA fragments or sequence many short DNA fragments more quickly (or both). We already noted the shotgun sequencing strategy used by Venter to sequence whole genomes (including our own—or, more accurately, his own!).
In shotgun sequencing, cloned DNA fragments of 1,000 bp or longer are broken down at random into smaller, more easily sequenced fragments. The fragments are themselves cloned and sequenced. Non redundant sequences are then assembled by the aligning of sequence-overlap regions.
When there are no gaps (which would be due to unrepresented DNA fragments that fail to be sequenced), today’s computer software is quite adept at rapidly aligning the available overlapping sequences and connecting them to display long, contiguous DNA sequences. Shotgun sequencing is summarized in Figure 15.19.

Sequence gaps that remain after shotgun sequencing can be filled in by primer walking. In this method, a sequencing primer (created based on a known sequence near the gap) “walks” into the gap region on an intact DNA (i.e., that has not been fragmented). Another “gap-filling” technique involves the polymerase chain reaction (PCR). Two oligonucleotides are synthesized, based on sequence information on either side of a gap; then, PCR is used to synthesize the missing fragment, which is sequenced to fill in the gap (PCR will be described more fully in section 15.5).
15.3.4 Next-Generation DNA Sequencing
The goals of next gen sequencing methods are to get an accurate reading of millions or even billions of bases in a short time. Examples of these sequencing systems include pyrosequencing, ligation-based sequencing, and ion-semiconductor sequencing; these involve methods of massively parallel sequencing reactions. They differ in detail, but all require a library (e.g., genomic, or transcriptomic) attached to a solid surface, not unlike a microarray. The attached DNAs are amplified in situ into separate DNA clusters derived from single library clones. The amplified, clustered DNAs then participate in the sequencing reaction. Sequences are analyzed and generated by computer. However, accuracy is a problem. Longer reads take more reactions and more time, and this leads to reduced accuracy. For a look at some of these technologies, see Next Generation Sequencing Video. A more accurate new technology is nanopore-based DNA sequencing, outlined in Figure 15.20. Nanopore sequencing was first used on the space station in 2016.

This device uses an artificial phospholipid membrane, in which is embedded a synthetic, engineered helicase/motor-pore-protein complex. The nanopore is itself derived from the Mycobacterium smegmatis porin A (MspA). A voltage is applied across the membrane to create a membrane potential. Double-stranded DNA is propelled into the helicase, where it is unwound. The resulting single-stranded molecules then pass through a tiny hole (the nanopore) in the MspA. As the ssDNA (or RNA) passes through, its charge configurations disturb the electrical field in the porin. These disturbances are different for each nucleotide, generating a unique signal that is detected, recorded, and displayed in almost real time on a small computer. Next-generation sequencing protocols and devices are already providing long and increasingly more accurate data, which is required for research and for clinical applications. Click Sequencing DNA in Space (again) to see why one would want to do this!
While first-generation automated dideoxy-Sanger sequencing is still widely used to analyze individual cDNAs, genes or PCR products, next-gen DNA sequencing is not only more rapid. What’s more, it can also be used in the field, for research as well as for practical analyses. And, as if this weren’t enough, now comes word of a marriage of a nano-sequencer and a cell phone analytical app to make immediate sense of a nano-sequence (iGenomics: A Nanotech Marriage). Check out your Star Trek creds and ask can a real tricorder be on the way to a real Dr. McCoy even before we have a real Enterprise? Will this new married couple be able to identify microbial agents of disease on location?


