6.11: Fatty Acid Oxidation
- Page ID
- 2998
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
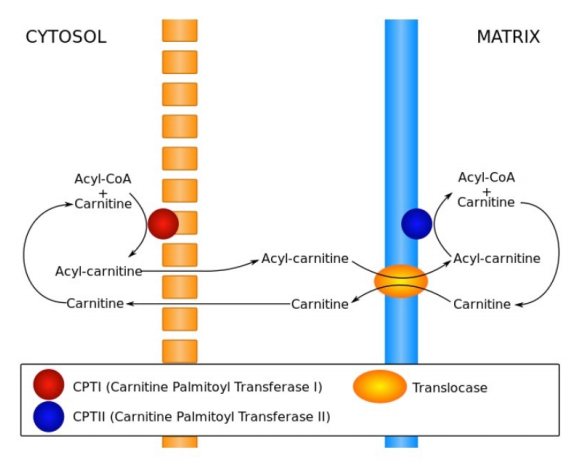
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Breakdown of fats yields fatty acids and glycerol. Glycerol can be readily converted to DHAP for oxidation in glycolysis or synthesis into glucose in gluconeogenesis. Fatty acids are broken down in two carbon units of acetyl-CoA. To be oxidized, they must be transported through the cytoplasm attached to coenzyme A and moved into mitochondria. The latter step requires removal of the CoA and attachment of the fatty acid to a molecule of carnitine. The carnitine complex is transported across the inner membrane of the mitochondrion after which the fatty acid is reattached to coenzyme A in the mitochondrial matrix.
The process of fatty acid oxidation, called beta oxidation, is fairly simple. The reactions all occur between carbons 2 and 3 (with #1 being the one linked to the CoA) and sequentially include the following:
- dehydrogenation to create \(\text{FADH}_2\) and a fatty acyl group with a double bond in the trans configuration;
- hydration across the double bond to put a hydroxyl group on carbon 3 in the L configuration;
- oxidation of the hydroxyl group to make a ketone; and
- thiolytic cleavage to release acetyl-CoA and a fatty acid two carbons shorter than the starting one.
Unsaturated fatty acids complicate the picture a bit (see below), primarily because they have cis bonds, for the most part, if they are of biological origin and these must be converted to the relevant trans intermediate made in step 1. Sometimes the bond must be moved down the chain, as well, in order to be positioned properly. Two enzymes (described below) handle all the necessary isomerizations and moves necessary to oxidize all of the unsaturated fatty acids.
Enzymes of Beta Oxidation
The reactions of fatty acid oxidation are notable in mirroring the oxidations in the latter half of the citric acid cycle – dehydrogenation of succinate to make a transdouble bond (fumarate), hydration across the double bond to make L-malate and oxidation of the hydroxyl to make a ketone (oxaloacetate). Two of the enzymes of beta-oxidation are notable. The first is acyl-CoA dehydrogenase, which catalyzes the initial dehydrogenation and yields FADH2. It comes in three different forms – ones that work on long, medium, or short chain length fatty acids. The first of these is sequestered in the peroxisome of animals whereas the others are found in the mitochondria. Plants and yeast perform beta oxidation exclusively in the peroxisome. The most interesting of the acyl-CoA dehydrogenases is the one that works on medium length fatty acids. This one, which is the one most commonly deficient in animals, has been linked to sudden infant death syndrome.
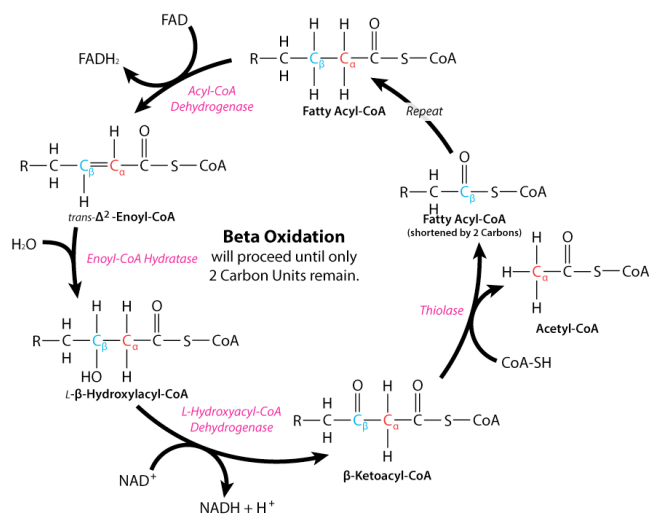
Reactions two and three in beta oxidation are catalyzed by enoyl-CoA hydratase and 3-hydroxyacyl-CoA dehydrogenase, respectively. The latter reaction yields an NADH. The final enzyme of beta oxidation is thiolase and this enzyme is notable in not only catalyzing the formation of acetyl-CoAs in beta oxidation, but also catalyzing the joining of two acetyl-CoAs (essentially the reversal of the last step of beta oxidation) to form acetoacetyl-CoA– essential for the pathways of ketone body synthesis and cholesterol biosynthesis.
Oxidation of Odd-Chain Fatty Acids
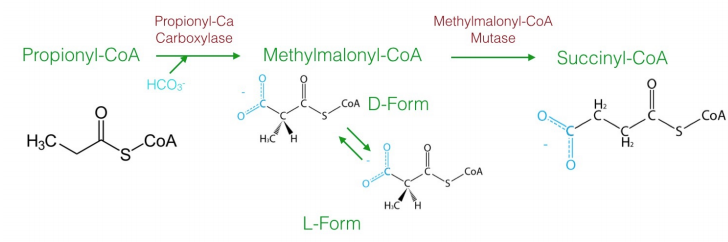
Though most fatty acids of biological origin have even numbers of carbons, not all of them do. Oxidation of fatty acids with odd numbers of carbons ultimately produces an intermediate with three carbons called propionyl-CoA, which cannot be oxidized further in the beta-oxidation pathway. Metabolism of this intermediate is odd. Sequentially, the following steps occur:
- carboxylation to make D-methylmalonyl-CoA;
- isomerization to L-methylmalonyl-CoA;
- rearrangement to form succinyl-CoA. The last step of the process utilizes the enzyme methylmalonyl-CoA mutase, which uses the \(\text{B}_12\) coenzyme in its catalytic cycle. Succinyl-CoA can then be metabolized in the citric acid cycle.
Unsaturated Fatty Acid Oxidation
As noted above, oxidation of unsaturated fatty acids requires two additional enzymes to the complement of enzymes for beta oxidation. If the beta oxidation of the fatty acid produces an intermediate with a cis bond between carbons three and four, cis-\(\Delta\)3-Enoyl-CoA Isomerase will convert the bond to a trans bond between carbons two and three and beta oxidation can proceed as normal.
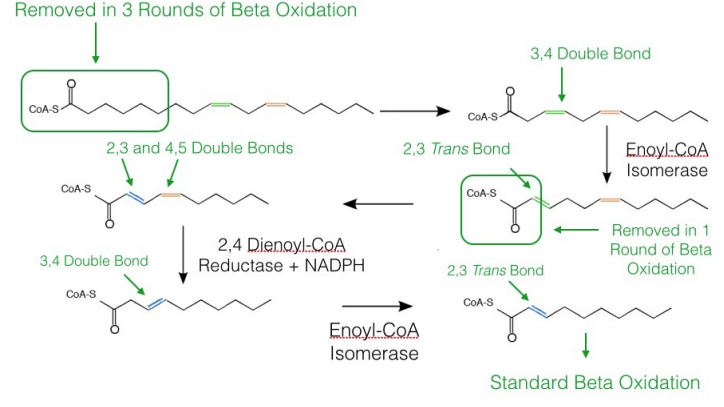
On the other hand, if beta oxidation produces an intermediate with a cis double bond between carbons four and five, the first step of beta oxidation (dehydrogenation between carbons two and three) occurs to produce an intermediate with a trans double bond between carbons two and three and a cis double bond between carbons four and five. The enzyme 2,4 dienoyl CoA reductase reduces this intermediate (using NADPH) to one with a single cis bond between carbons three and four. This intermediate is then identical to the one acted on by cis-\(\Delta\)3-Enoyl-CoA Isomerase above, which converts it into a regular beta oxidation intermediate, as noted above.
Alpha Oxidation
Yet another consideration for oxidation of fatty acids is alpha oxidation. This pathway is necessary for catabolism of fatty acids that have branches in their chains. For example, breakdown of chlorophyll’s phytol group yields phytanic acid, which undergoes hydroxylation and oxidation on carbon number two (in contrast to carbon three of beta oxidation), followed by decarboxylation and production of a branched intermediate that can be further oxidized by the beta oxidation pathway. Though alpha oxidation is a relatively minor metabolic pathway, the inability to perform the reactions of the pathway leads to Refsum’s disease where accumulation of phytanic acid leads to neurological damage.


