9.5: Fatty Acid Oxidation
- Page ID
- 66029
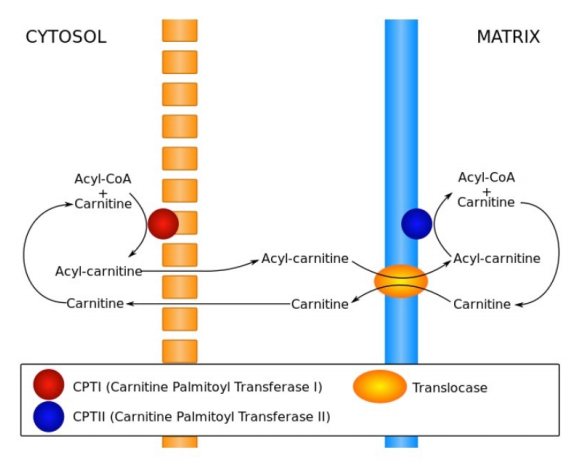
The process of fatty acid oxidation, called beta oxidation, is fairly simple. The reactions all occur between carbons 2 and 3 (with #1 being the one linked to the CoA) and sequentially include the following:
- dehydrogenation to create \(\text{FADH}_2\) and a fatty acyl group with a double bond in the trans configuration;
- hydration across the double bond to put a hydroxyl group on carbon 3 in the L configuration;
- oxidation of the hydroxyl group to make a ketone; and
- thiolytic cleavage to release acetyl-CoA and a fatty acid two carbons shorter than the starting one.
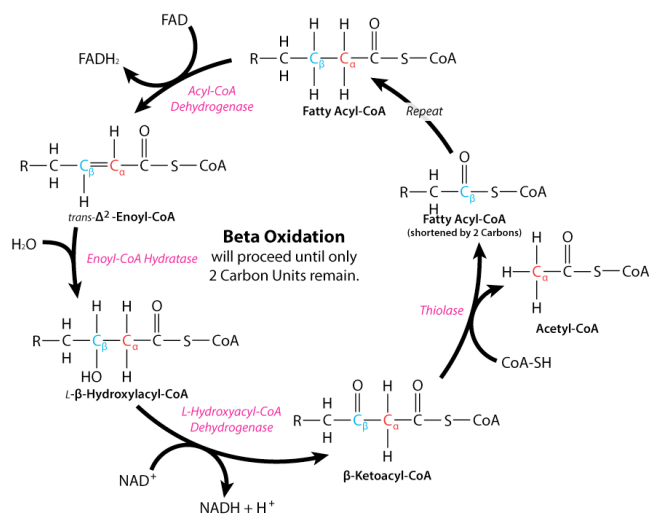
Reactions two and three in beta oxidation are catalyzed by enoyl-CoA hydratase and 3-hydroxyacyl-CoA dehydrogenase, respectively. The latter reaction yields an NADH. The final enzyme of beta oxidation is thiolase and this enzyme is notable in not only catalyzing the formation of acetyl-CoAs in beta oxidation, but also catalyzing the joining of two acetyl-CoAs (essentially the reversal of the last step of beta oxidation) to form acetoacetyl-CoA– essential for the pathways of ketone body synthesis and cholesterol biosynthesis.
Oxidation of Odd-Chain Fatty Acids
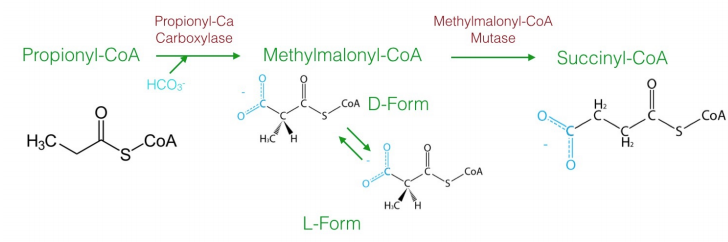
Though most fatty acids of biological origin have even numbers of carbons, not all of them do. Oxidation of fatty acids with odd numbers of carbons ultimately produces an intermediate with three carbons called propionyl-CoA, which cannot be oxidized further in the beta-oxidation pathway. Metabolism of this intermediate is odd. Sequentially, the following steps occur:
- carboxylation to make D-methylmalonyl-CoA;
- isomerization to L-methylmalonyl-CoA;
- rearrangement to form succinyl-CoA. The last step of the process utilizes the enzyme methylmalonyl-CoA mutase, which uses the \(\text{B}_12\) coenzyme in its catalytic cycle. Succinyl-CoA can then be metabolized in the citric acid cycle.
On the other hand, if beta oxidation produces an intermediate with a cis double bond between carbons four and five, the first step of beta oxidation (dehydrogenation between carbons two and three) occurs to produce an intermediate with a trans double bond between carbons two and three and a cis double bond between carbons four and five. The enzyme 2,4 dienoyl CoA reductase reduces this intermediate (using NADPH) to one with a single cis bond between carbons three and four. This intermediate is then identical to the one acted on by cis-\(\Delta\)3-Enoyl-CoA Isomerase above, which converts it into a regular beta oxidation intermediate, as noted above.
Contributors and Attributions
Dr. Kevin Ahern and Dr. Indira Rajagopal (Oregon State University)


