2017_SS1_Lecture_01
- Page ID
- 9738
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Biology
Biology is the scientific study of life. Studying biology is an opportunity to ask exciting questions about the world that surrounds us. It is an opportunity to dig into some of humanity's deepest questions about our origins, our planet's history, and our connections to other living beings (big and small/extant or extinct). It is also an opportunity to dive into a world of practical problem solving and to think hard about possible solutions for improving health care, maintaining sustainable food supplies, and producing renewable energy technologies.
Studying biology helps us understand issues and address everyday problems. For instance, you can better understand how what you eat and the amount you exercise influence your health when you understand the biochemical reactions that describe how the food (matter) is transformed, how it and your body store energy, and how this energy can be transferred from the food to your muscles. Deciding whether or not to buy products labeled with terms like "antimicrobial" or "probiotic" can be easier if you understand what the microbes, which live in, on, and around us, do. Understanding the biochemical principles that describe the changes that happen to eggs as they cook can help us understand how similar physical processes may be central to the cellular stress response and some diseases. Your eye color can be better appreciated with an understanding of the genetic and biochemical mechanisms that link genetic information to physical traits.
Studying biology even helps us understand things that are "out of this world." For instance, understanding the requirements for life can help us look for life in places like Mars or deep in Earth’s crust. When we understand how to properly “rewire” cellular decision-making networks, we may finally be able to regenerate functional limbs or organs from someone’s own tissue, or reprogram diseased tissues back to health. There are many exciting opportunities. The key point is that mastering a few basic principles helps you understand and think more deeply about a wide array of topics. Keep this notion in mind throughout the course.
Biology: an interdisciplinary science
Questions in biology span size scales in excess of ten orders of magnitude, from the atomic makeup and chemical behavior of individual molecules to planetary-scale systems of interacting ecologies. Whatever the scale of interest, to develop a deep and functional understanding of biology, we must first appreciate biological concepts. This involves integrating important ideas and tools from across the spectrum of science, including chemistry, physics, and mathematics. Biology is truly an interdisciplinary science.
The potential application of knowledge is broad
Some people may think studying biology is only about medicine—however, it can lead to or influence many different careers. Biology has applications that are both vast and wide-ranging. Applications include treating (human or other animal) patients, improving agricultural practices, developing new building materials, writing new energy policies, remedying global climate change, creating new works of art—the list goes on and on. For the curious, biology has plenty of unexplored mysteries.
As you study biology, appreciate its exciting questions and topics and be open-minded. Even though course topics may not always seem related at first, they likely are. Being open-minded helps you discover and appreciate the connections between the course’s topics and your interests. Discovering how seemingly different topics interrelate can give you a deeper appreciation for the things you enjoy and maybe even spark a new passion.
BIS2A—from molecules to cells
BIS2A focuses on the cell, one of the most fundamental units of life. Cells can be as simple as the disease-causing bacterium Mycoplasma genitalium, whose genome encodes just 525 genes (only 382 of which are essential for life), or as complex as a cell belonging to the multicellular plant Oryza sativa (rice), whose genome likely encodes ~51,000 genes. However, in spite of this diversity, all cells share some fundamental properties. In BIS2A, we explore basic problems that must be dealt with by all cells. We study the building blocks of cells, some of their key biochemical properties, how biological information is encoded and expressed in genetic material, and how all this combines to make a living system. We will also discuss some of the ways in which living systems exchange matter, energy, and information with their environment (including other living things). We focus primarily on core principles that are common to all life on Earth, and due to biology's large breadth, we put these ideas into a variety of contexts throughout the quarter.
BIS2A
Biological Sciences 2A at UC Davis. BIS2A is a 5-unit course with either three 50 minute or two 1 hour and 50 minute lectures (depending on the quarter) plus a 2-hour discussion each week. BIS2A is the first of three courses in the lower division core sequence in the biological sciences. BIS2A provides a foundation in key biological concepts that are of use across a broad spectrum of majors. Students are introduced to the fundamental chemical, molecular, genetic, and cellular building blocks of life, biological mechanisms for the recruitment and transfer of matter and energy, basic principles of biological information flow and cellular decision making, and core concepts underlying the relationships between genetic information and phenotype.
It is important to realize that BIS2A is not a survey course in biology. Biology is an exciting, broad, and dynamic field. It is critical for students in biology or related fields to develop a strong conceptual foundation and to demonstrate their ability to use it in contexts that may be novel to them. Students in BIS2A will be asked to begin developing the ability to identify and articulate the key scientific and biological questions that are at the core of the course content. Students will be expected to learn and use correct technical vocabulary in their discussions of course content. Students will be expected to begin conceptualizing course content from a question-driven and problem-solving perspective.
Yes, BIS2A will require you to work hard, but we also hope that you will have fun discovering new aspects of biology and exploring the many unanswered questions concerning what it means to be alive.
The main course learning objectives include:
- Apply principles of chemistry and bioenergetics in the context of biological systems to describe how cells acquire and transform matter and energy to build and fuel various life sustaining processes, including chemical transformations of elemental compounds, cellular replication, and cellular information processing.
- Explain the relationship between genotype and key genetic processes that create phenotypic diversity.
- Describe the processes regulating the management of cellular information; how information is stored, read, rearranged, replicated; how cells interact with their environment and how these processes can control cellular physiology.
Who should I ask when I have questions about the course?
- General information about the course: The syllabus provides most of this type of information. For the quickest answers to many of your questions, we highly recommend looking at the syllabus before contacting one of the staff.
- General information about topics in BIS2A: The BIS2A Learning Center (BLC), which is in RM 2089 SLB, is a resource center for all BIS2A students. The BLC is staffed by the instructors and teaching assistants associated with all BIS2A sections. Any BIS2A instructor or TA having office hours in the BLC should be able to answer general questions about the lecture and discussion material. If they can’t answer your questions, they will be happy to refer you to someone who can.
- Lecture material and Nota Bene assignments: Your Lecture TA is a great source of information about the lecture material and any lecture related reading specific to your section of BIS2A.
- Discussion material: Your discussion TA is the best source of information about the discussion material present in your specific discussion section.
- All course content related material: Your instructor is a great resource for questions about course related material. Find your instructor after class and go to their office hours whenever possible.
Some of your responsibilities
BIS2A is a team effort. Several professors are involved in developing the course content and assessment materials. There are also teaching assistants, who not only run the discussion sections, but also provide insights into which concepts students find the most difficult.
Please keep up with your responsibilities as a student. Do the assigned reading and start to learn new vocabulary before coming to class. Come to class prepared to engage - your instructor will assume that you have read the material before class and that the lecture will not be your first exposure to the content. After class, review your notes, the podcast, and the post-study guide. Seek out assistance immediately when you need it. If everyone in the class can conscientiously do these things, we’ll all have fun this quarter (even while working hard) and be a happy and smarter bunch at the end of the term!
Active learning in BIS2A
In every lecture, we will ask you to answer questions, either in a small group or individually. These questions serve several purposes:
Functions of in-class questions
-
Questions stimulate students to examine a topic from a different perspective, one that the instructor considers relevant to their learning.
-
Questions act as mini "self-tests" for students. If you are uncertain about what question is being asked or how to answer it, this is a good time to (a) ask the instructor for clarification and/or (b) take note to review this immediately after class with a TA, the instructor, classmates, or the internet. If the instructor took the time to ask you the question in class, this is a big clue that he/she thinks that both the question and the answer are important.
-
Some in-class questions will ask students to formulate questions themselves. This is typically an exercise that is designed to force the student to reflect on and try to articulate the point of the lesson. These are critical exercises that force you to think more deeply about a topic and to place it in the broader context of the course.
-
Some questions may ask the student to interpret data or to create a model (e.g., perhaps a picture) and to communicate what they see to the class. This exercise asks the student to practice explaining something out loud. This can be a great self-test and learning experience, both for the person answering and fellow students who should also be using the time to examine how they would have answered the question and how that compares with the feedback of the instructor.
-
Questions in the discussion that follows and the thought process involved in solving a problem or answering the questions are opportunities for the instructor to model expert behavior in an interactive way—sometimes it is equally important to understand HOW we arrive at an answer as it is to understand the answer.
Some questions are designed to stimulate thought and discussion rather than to elicit a discrete answer. If called on, you should not feel compelled to have one "right" answer!! Understanding this is very important. Once you realize that it is perfectly acceptable (and sometimes desirable) to not know all of the answers (if you did, what would be the point of coming to class), it can take away a lot of the anxiety of getting called on. While it is okay to not know "the answer", it is nevertheless important for you to attempt to make a contribution to the discussion. Examples of other meaningful contributions might include: asking for clarification; associating the question with another class topic (trying to make connections); and expressing what you are comfortable with and what confuses you about the question. Don't be afraid to say "I don't know". That's perfectly okay and even expected sometimes. Be prepared for the instructor to follow up with a different question, however, that will try to either highlight something that you likely do know or to ask for your help with identifying a point of confusion.
Getting ready for lecture
To help you get ready for each lecture, we provide study guides that include instructions on how to prepare for class. You should do your best to complete the assigned reading and suggested "self-assessments" before coming to class. This will ensure that you are ready for discussions and that you can make the most of your time during class. We do not expect you to be an expert before lecture, but we do expect you to do the pre-reading and by doing so make yourself familiar with the required vocabulary and spend some time thinking about the concepts that will be discussed. We will build on that basic knowledge in lecture. If you do not have at least some of the basic building blocks before hand, you will make less efficient use of your time in class.
We cannot emphasize too strongly that YOU have the primary responsibility for learning the material in this (or any other) course. Although we are invested in your success, your instructors and TAs cannot magically implant knowledge. Like any other discipline that requires mastery (e.g., sports, music, dance, etc.), we can help guide you and critique your performance, but we can not replace the hours of practice necessary to become good at something. You would never expect to become a proficient pianist by going to lessons once or twice a week and never practicing. To most of us, it seems self-evident that you need practice to become good at something like music, art, or sports. It should not be surprising that the same rule applies with learning biology or any other academic subject.
We see ourselves as your coaches for this class; we want all of you to succeed. However, for this to happen, you have to take your practice seriously. This means coming to class prepared, participating in class, studying the material covered in class as soon as possible, identifying where you are uncertain and getting help to clarify those topics as soon as possible, and trying to make thoughtful contributions to the online discussions (not just the bare minimum required to "get the points").
Bottom line: you need to be active participants in your learning.
Knowledge and Learning
Teaching and Learning Science
Teaching and learning science are both challenging endeavors. As instructors, we need to communicate complex, highly interconnected concepts that will serve as a foundation for all your future studies. We also want our students to demonstrate mastery of these ideas at a high level. As students, you need to learn a large new vocabulary, create mental models on which you can "hang" the new conceptual knowledge, and demonstrate that you can actually use this new knowledge. The process challenges both the instructor and the student. Although the process involves hard work, it can also be incredibly rewarding. There is nothing more satisfying for an instructor than those “Aha!” moments when a student suddenly understands an important concept.
In BIS2A we face some interesting teaching and learning challenges. One key challenge is that we discuss physical things and ideas that exist or happen on time and/or size scales that are not familiar to most students. What does this mean? Consider the following example:
Example: Some challenges associated with creating mental models
An instructor teaching wildlife biology may want to talk about concepts in evolution by using bird beaks as a starting point for discussion. In this case, the instructor does not need to spend time creating mental pictures of different shaped bird beaks (or at the very least only needs to show one image); most students will readily draw on their past knowledge and everyday lives to create mental pictures of duck, eagle, or wood pecker beaks and infer the different functional reasons why Nature might have selected different shapes. As a consequence, the students will not need to expend any mental effort imagining what the beaks look like and can instead focus all of their energies on the core evolutionary lesson.
More colloquially: If you are asked to think about something new that is closely related to something you already know well, it is not too difficult to focus on the new material.
By contrast, in BIS2A we ask students to think about and discuss things that happen on the atomic, molecular and cellular scales and at rates that span microseconds to millennia. Most students, we will guess, have not lived life on the micro to nanometer scale. Yet, this length scale is where most of the events common to all biological systems takes place. Beginning students, who have not thought much about how things happen at the molecular scale, lack mental models upon which to add new information. This starting point places a burden on both the student and the instructors to create and reinforce NEW mental models for many of the things we talk about in class. For instance, to really talk about how proteins function, we first need to develop a common set of models and vocabulary for representing molecules at the atomic and molecular levels. Not only do these models need to find ways of representing the molecule’s structure, but the models must also contain abstract ideas about the chemical properties of molecules and how these molecules interact. Therefore, students in BIS2A need to put some effort into constructing mental models of what proteins "look" like and how they behave at the molecular scale. Since the entire course centers around biomolecules and processes that happen at a microscopic scale, a similar argument can be made for nearly every topic in the class.
Note: Possible Discussion
How do you interpret the term mental model and why do you think that it is important for learning?
Some of the in-class and study guide exercises are designed to help with meeting this challenge; most students have found them very useful. However, some students are more accustomed to studying for exams by memorizing information rather than understanding it. (It's not their fault; that's what they were asked to do in the past). As a result, if the problems are approached with the "memorize-at-all-costs" attitude some of the BIS2A exercises may initially seem pointless. For instance, why are your instructors asking you to repeatedly draw some of the concepts described in class? What multiple-choice question could that exercise possibly prepare you for? While it is true that some of your instructors won't ask you to draw complicated figures on an exam, these drawing exercises are not trying to prepare students for one specific question. Rather the instructor is trying to encourage you to begin creating a mental model for yourself and to practice using it. The act of drawing can also serves as a "self test." When you force yourself to write something down or to create a picture describing a process on paper, you will be able to independently assess how strong your conceptual grasp of a topic really is by seeing how easy or hard it was to put your mental image of something onto paper. If it is hard for you to draw a core concept or process from class WITHOUT EXTERNAL ASSISTANCE, it is likely that you need more practice. If it is easy, you are ready to add new information to your model. Throughout the course, you will continue to add new information to your mental model or to use the concept represented in your mental model in a new context. Keep your drawings - or other self-testing mechanisms - current. Don't fall behind.
Incidentally, the presentation of a course concept on an exam in a context that the student has never seen before is NOT an evil plot by the instructor. Rather it is a way for the instructor and student to assess whether the concept has been learned and whether that knowledge can be used/transferred by the student outside of the specific example given in class or in the reading. Asking the student to repeat the latter would represent an exercise in memorization and would not be an assessment of valuable learning and independent thinking or a representation of what happens in real life.
IMPORTANT: The idea that students in BIS2A will be tested on their ability to USE concepts in specific contexts that they haven't seen before is critical to understand! Take special heed of this knowledge. Developing usable conceptual knowledge takes more discipline and work than memorizing. The quarter also moves VERY fast and concepts are layered one on top of the other. If you get too far behind, it is very, very difficult to make up for lost time two or three days before an exam. Be as disciplined as you can and keep up with course materials.
So, some concepts are hard to teach and to understand. What are we to do? Something instructors and students both do is to use various communication tricks to simplify or make abstract ideas more relatable. We use tools like analogies or simplified models (more on the importance of these shortly) to describe complex ideas. Making things more relatable can take various forms. Instructors might try to use various simlies or metaphors to take advantage of mental pictures or conceptual models that students already have (drawn from everyday life) to explain something new. For instance, the thing X that you don't understand works a little like thing Y that you do understand. Sometimes, this helps ground a discussion. Another thing you might catch an instructor or student doing is anthropomorphizing the behaviors of physical things that are unfamiliar. For example we might say molecule A “wants" to interact with molecule B to simplify the more correct but more complex description of the chemical energetics involved in the interaction between molecules A and B. Anthropomorphisms can be useful because, like similes and metaphors, they attempt to link the creation of new ideas and mental models to concepts that already exist in the student's brain.
While these tools can be great and effective they nevertheless need to be used carefully - by both the instructor and the student. The main risk associated with these simplifying tools is that they can create conceptual connections that shouldn't exist, that lead to unintended misconceptions, or that makes it more difficult to connect a new concept. So while these tools are valid, we - students and instructors - also need to be vigilant about understanding the limits these tools have in our ability to learn new ideas. If these pedagogical tools are useful but their use also carries risk, how do we proceed?
The remedy has two parts:
1. Recognize when one of these "simplifying" tools is being used and
2. Try to determine where the specific analogy, metaphor etc. works and where it fails conceptually.
The second instruction is the most difficult and may prove challenging for learners, particularly when they are first exposed to a new concept. However, the act of simply thinking about the potential problems associated with an analogy or model is an important metacognitive exercise that will help students learn. In BIS2A your instructors will occasionally expect you to explicitly recognize the use of these pedagogical tools and to explain the trade-offs associated with their use. Your instructors will also help you with this by explicitly pointing out examples or prodding you to recognize a potential issue.
Note: Possible Discussion
Can you give an example from your previous classes where an instructor has used an anthropomorphism to describe a nonhuman thing? What were/are the trade-offs of the description (i.e. why did the description work and what were its limitations)?
Using vocabulary
It is also worth noting another problematic issue that can needlessly confound students just starting out in a discipline - the use of vocabulary terms that potentially have multiple definitions and/or the incorrect use of vocabulary terms that have strict definitions. While this is not a problem unique to biology, it is nevertheless important to recognize that it occurs. We can draw from real-life examples to get a better sense of this issue. For instance, when we say something like "I drove to the store", a couple of things are reasonably expected to be immediately understood. We don't need to say "I sat in and controlled a four-wheeled, enclosed platform, that is powered by the combustion of fossil fuel to a building that collects goods I want to obtain and can do so by exchanging fungible currency for said goods" to convey the core of our message. The downside to using the terms "drove" and "store" is that we have potentially lost important details about what really happened. Perhaps the car is battery powered and that is important to understanding some detail of the story that follows (particularly if that part of the story involves calling a tow truck driver to pick you up after the car has broken down). Perhaps knowing the specific store is important for understanding context. Sometimes those details don't matter, but sometimes if they aren’t known it can lead to confusion. Using vocabulary correctly and being careful about word choice is important. Knowing when to simplify and when to give extra detail is also key.
Aside:
In the laboratory, undergraduate students in biology will often report back to their mentors that "my experiment worked" without sharing important details of what it means to have "worked", what the evidence is, how strong the evidence is, or what the basis is for their judgment - all details that are critical to understanding exactly what happened. If and/or when you start working in a research lab do yourself and your advisor the favor of describing IN DETAIL what you were trying to accomplish (don't assume they'll remember the details), how you decided to accomplish your goal (experimental design), what the exact results were (showing properly labeled data is advised), and providing your interpretation. If you want to end your description by saying "therefore, it worked!" that's also great.
Note: Possible Discussion
Can you think of an example where the imprecise or incorrect use of vocabulary caused needless confusion in real life? Describe the example and discuss how the confusion could have been avoided.
Models and simplifying assumptions
Creating models of real things
Life is complicated. To help us understand what we see around us—in both our everyday lives and in science or engineering—we often construct models. A common aphorism states: all models are wrong, but some are useful. That is, no matter how sophisticated, all models are approximations of something real. While they are not the “real thing” (and are thus wrong), models are useful when they allow us to make predictions about real life that we can use. Models come in a variety of forms that include, but are not limited to:
Types of models
- Physical models: These are 3-D objects that we can touch.
- Drawings: These can be on paper or on the computer and either in 2-D or virtual 3-D. We mostly look at them.
- Mathematical models: These describe something in real life in mathematical terms. We use these to calculate the behavior of the thing or process we want to understand.
- Verbal or written models: These models are communicated in written or spoken language.
- Mental models: These models are constructed in our minds and we use these to create the other types of models and to understand the things around us.
Simplifying assumptions
Usually, in science and everyday life alike, simple models are preferred over complex ones. Creating simple models of complex real things requires us to make what are known as simplifying assumptions. As their name implies, simplifying assumptions are assumptions that are included in the model to simplify the analysis as much as possible. When a simplified model no longer predicts behavior of the real thing within acceptable bounds, too many simplifying assumptions have been made. When little predictive value is gained from adding more details to a model, it is likely overly complex. Let’s take a look at different types of models from different disciplines and point out their simplifying assumptions.
An example from physics: a block on a frictionless plane
Figure 1. A line drawing that models a block (of any material) sitting on a generic incline plane. In this example some simplifying assumptions are made. For instance, the details of the materials of the block and plane are ignored. Often, we might also, for convenience, assume that the plane is frictionless. The simplifying assumptions allow the student to practice thinking about how to balance the forces acting on the block when it is elevated in a gravity field and to see that the surface it is sitting on is not perpendicular to the gravity vector (mg). This simplifies the math and allows the student to focus on the geometry of the model and how to represent that mathematically. The model, and its simplifying assumptions, might do a reasonably good job of predicting the behavior of an ice cube sliding down an glass incline plane but would likely do a bad job of predicting the behavior of a wet sponge on an incline plane coated with sand paper. The model would be oversimplified for the latter scenario.
Source: Created by Marc T. Facciotti (Own work)
An example from biology: a ribbon diagram of a protein—the transmembrane protein bacteriorhodopsin
Figure 2. This is a cartoon model of the transmembrane protein bacteriorhodopsin. The protein is represented as a light blue and purple ribbon (the different colors highlight alpha helix and beta sheet, respectively), a chloride ion is represented as a yellow sphere, red spheres represent water molecules, pink balls and sticks represent a retinal molecule located on the "inside" of the protein, and orange balls-and-sticks represent other lipid molecules located on the "outside" surface of the protein. The model is displayed in two views. On the left the model is viewed "side on" while on the right it is viewed along its long axis from the extracellular side of the protein (rotated 90 deg out of the page from the view on the left). This model simplifies many of the atomic-level details of the protein. It also fails to represent the dynamics of the protein. The simplifying assumptions mean that the model would not do a good job predicting the time it takes for the protein to do its work or how many protons can be transported across a membrane per second. On the other hand, this model does a very good job of predicting how much space the protein will take up in a cellular membrane, how far into the membrane the retinal sits, or whether certain compounds can reasonably “leak” through the inner channel.
Source: Created by Marc T. Facciotti (own work), University of California, Davis
Derived from PDBID:4FPD
An example from chemistry: a molecular line model of glucose
Figure 3. A line drawing of a glucose molecule. By convention, the points where straight lines meet are understood to represent carbon atoms while other atoms are shown explicitly. Given some additional information about the nature of the atoms that are figuratively represented here, this model can be useful for predicting some of the chemical properties of this molecule, including solubility or the potential reactions it might enter into with other molecules. The simplifying assumptions, however, hide the dynamics of the molecules.
Source: Created by Marc T. Facciotti (Own work)
An example from everyday life: a scale model of a Ferrari
Figure 4. A scale model of a Ferrari. There are many simplifications and most only make this useful for predicting the general shape and relative proportions of the real thing. For instance, this model gives us no predictive power about how well the car drives or how quickly it stops from a speed of 70 km/s.
Source: Created by Marc T. Facciotti (Own work)
Note: possible discussion
Describe a physical model that you use in everyday life. What does the model simplify from the real thing?
Note: possible discussion
Describe a drawing that you use in science class to model something real. What does the model simplify from the real thing? What are the advantages and disadvantages of the simplifications?
The spherical cow
The spherical cow is a famous metaphor in physics that make fun of physicists tendencies to create hugely simplified models for very complex things. Numerous jokes are associated with this metaphor and they go something like this:
"Milk production at a dairy farm was low, so the farmer wrote to the local university, asking for help from academia. A multidisciplinary team of professors was assembled, headed by a theoretical physicist, and two weeks of intensive on-site investigation took place. The scholars then returned to the university, notebooks crammed with data, where the task of writing the report was left to the team leader. Shortly thereafter the physicist returned to the farm, saying to the farmer, "I have the solution, but it only works in the case of spherical cows in a vacuum"."
Source: Wikipedia page on Spherical Cow - accessed November 23, 2015.
Figure 5. A cartoon representation of a spherical cow.
Source: https://upload.wikimedia.org/wikiped.../d2/Sphcow.jpg
By Ingrid Kallick (Own work) [GFDL (http://www.gnu.org/copyleft/fdl.html) or CC BY 3.0 (http://creativecommons.org/licenses/by/3.0)], via Wikimedia Commons
The spherical cow is an amusing way to ridicule the process of creating simple models and it is quite likely that you will have your BIS2A instructor invoke the reference to the spherical cow when an overly simplified model of something in biology is being discussed. Be ready for it!
Bounding or asymptotic analysis
In BIS2A, we use models frequently. Sometimes we also like to imagine or test how well our models actually represent reality and compare that with expectations from what we know to be true for the real life thing. There are many ways to do this depending on how precisely you need to know the behavior of the thing you're trying to model. If you need to know a lot of detail, you create a detailed model. If you're willing to live with less detail, you will create a simpler model. In addition to applying simplifying assumptions, it is often useful to assess your model using a technique we call bounding or asymptotic analysis. The main idea of this technique is to use the model, complete with simplifying assumptions, to understand how the real thing might behave at extreme conditions (e.g., evaluate the model at the minimum and maximum values of a variable). Let’s examine a simple real life example of how this technique works.
Example: bounding
Problem setup
Imagine that you need to leave Davis, CA and get home to Selma, CA for the weekend. It's 5PM and you told your parents that you'd be home by 6:30. Selma is 200 miles (322 kM) from Davis. You're getting worried that you won't make it home on time. Can you get some estimate of whether it's even possible or if you'll be reheating your dinner in the microwave?
Create simplified model and use of bounding
You can create a simplified model. In this case you can assume that the road between Davis and Selma is perfectly straight. You also assume that your car has only two speeds: 0 mph and 120 mph. These two speeds are the minimum and maximum speeds that you can travel—the bounding values. You can now estimate that even under assumptions of the theoretically "best case" scenario, where you would drive on a perfectly straight road with no obstacles or traffic at maximum speed, you will not make it home on time. At maximum speed you would only cover 180 of the required 200 miles in the 1.5 hours you have.
Interpretation
In this real life example a simplified model is created. In this case, one very important simplifying assumptions is made: the road is assumed to be straight and free of obstacles or traffic. These assumptions allow you to reasonably assume that you could drive this road at full speed the whole distance. The simplifying assumptions simplified out a lot of what you know is actually there in the real world that would influence the speed you could travel and by extension the time it would take to make the trip. The use of bounding—or calculating the behavior of at the minimum and maximum speeds—is a way of making quick predictions about what might happen in the real world.
We will conduct similar analyses in BIS2A.
The importance of knowing key model assumptions
Knowing what simplifying assumptions are made in a model is critical to judging how useful it is for predicting real life and for starting to make a guess about where the model needs improving if it is not sufficiently predictive. In BIS2A you will periodically be asked to create different types of models and to explicitly identify the simplifying assumptions and the impact of those assumptions on the utility and predictive ability of the model. We will also use models together with bounding exercises to try learning something about the potential behavior of a system.
The scientific method: overview
An example of oversimplification that confounds many students of biology (particularly early in their studies) is the use of language that hides the experimental process used to build knowledge. For the sake of expediency, we often tell stories about biological systems as if we are presenting unquestionable facts. However, while we often write and speak about topics in biology with a conviction that gives the appearance of "factual" knowledge, reality is often more nuanced and filled with significant uncertainties. The "factual" presentation of material (usually lacking discussion of evidence or confidence in the evidence) plays to our natural tendency to feel good about "knowing" things, but it tends to create a false sense of security in the state of knowledge and does little to encourage the use of imagination or the development of critical thinking.
A better way to describe our knowledge about the natural world would be to explicitly qualify that what we know to be "true" in science represents only our current best understanding of a topic; an understanding that has not yet been refuted by experiment. Unfortunately, repeated qualification becomes rather cumbersome. The important thing to remember is that while we may not say so explicitly, all of the knowledge we discuss in class represents only the best of our current understanding. Some ideas have withstood repeated and varied experimentation while other topics have yet to be tested as thoroughly. So if we're not as certain about things as we'd like to believe sometimes, how do we know what to put confidence in and what to be skeptical of? The complete answer is non-trivial but it begins with developing an understanding of the process we use in science to build new knowledge. The scientific method is the process by which new knowledge is developed. While the process can be described with long lists of "steps" (often seen in textbooks), its core elements can be described more succinctly.
Succinct description of scientific method (adapted from Feynman)
- Make an observation about the world.
- Propose a possible explanation for the observation.
- Test the explanation by experiment.
- If the explanation disagrees with experiment, the explanation is wrong.
At its core, that's it! In science there may be multiple, simultaneously proposed explanations or ideas that are tested by experiment. The ideas that fail experimentation are left behind. The ideas that survive experimentation move forward and are often retested by alternative experiments until they too either fail or continue to be retained.
Making an observation and asking a question
The ability to make useful observations and/or ask meaningful questions requires curiosity, creativity, and imagination—this cannot be overstated. Indeed, historically, it is first and foremost the application of these skills, perhaps more than technical ability, which has led to big advances in science. Many people think that making meaningful observations and asking useful questions is the easiest part of the scientific method. This is not always the case. Why? Seeing what others have not yet asked and creativity take work and thoughtful reflection! In addition, our senses of observation are often biased by life experience, prior knowledge, or even our own biology. These underlying biases influence how we see the world, how we interpret what we see, and what we are ultimately curious about. This means that when we look at the world, we can miss a lot of things that are actually right under our noses. Douglas Adams, who is best known for his book entitled The Hitchhiker’s Guide to the Galaxy, once expanded on this point by writing:
“The most misleading assumptions are the ones you don't even know you're making.”
Scientists, therefore, need to be aware of any underlying biases and any assumptions that may influence how they internalize and interpret observations. This includes approaching our bias that the variety of places we get our knowledge (i.e., textbooks, instructors, the Internet) are representing the absolute truth with a healthy dose of skepticism. We need to learn to examine the evidence underling the “facts” we supposedly know and make critical judgments about how much we trust that knowledge. More generally, taking the time to make careful observations and to uncover any assumptions and biases that could influence how they are interpreted is, therefore, time well spent. This skill, like all others, needs to be developed and takes practice and we’ll try to start you on this in BIS2A.
For fun, and to test your observation skills, Google “observation tests”. Many of the search results will take you to interesting psychological tests and/or videos that illustrate how difficult accurate observation can be.
Generating a testable hypothesis
The "possible explanation" referred to in step three above has a formal name; it is called a hypothesis. A hypothesis is not a random guess. A hypothesis is an educated (based on prior knowledge or a new viewpoint) explanation for an event or observation. It is typically most useful if a scientific hypothesis can be tested. This requires that the tools to make informative measurements on the system exist and that the experimenter has sufficient control over the system in question to make the necessary observations.
Most of the time, behaviors of the system that the experimenter wants to test can be influenced by many factors. We call the behaviors and factors dependent and independent variables, respectively. The dependent variable is the behavior that needs explaining while the independent variables are all of the other things that can change and influence the behavior of the dependent variable. For example, an experimenter that has developed a new drug to control blood pressure may want to test whether her new drug actually influences blood pressure. In this example, the system is the human body, the dependent variable might be blood pressure, and the independent variables might be other factors that change and influence blood pressure like age, sex, and levels of various soluble factors in the blood stream.
Note: for more on dependent and independent variables
Note
In BIS2A, and beyond, we prefer to avoid using language like “the experiment proved her hypothesis” when referring to a case like the blood pressure example above. Rather we would say, “the experiment is consistent with her hypothesis.” Note that for convenience, we referred to the alternative hypothesis simply as “her hypothesis”! It would be more correct to state, “the experiment falsified her null hypothesis and is consistent with her alternative hypothesis.” Why take this shortcut since doing so adds confusion when a student is trying to learn? In this case, it was done to illustrate the point above about language shortcuts and hence the lengthy explanation. However, be aware of this commonly used shortcut and learn to make sure you can read in the correct meaning yourself.
Note: possible discussion
What does the statement about falsifying hypotheses mean in your own words? Why is falsification critical to the scientific method?
Controls
In an ideal case, an experiment will include control groups. Control groups are experimental conditions in which the values of the independent variables (there may be more than one) are maintained as close to those in the experimental group with the exception of the independent variable being tested. In the blood pressure example, an ideal scenario would be to have one identical group of people taking the drug and another group of people identical to those in the experimental group taking a pill containing something known to not influence blood pressure. In this oversimplified example, all independent variables are identical in the control and experimental groups with the exception of the presence or absence of the new drug. Under these circumstances, if the value of the dependent variable (blood pressure) of the experimental group differs from that of the control group, one can reasonably conclude that the difference must be due to the difference in independent variable (the presence/absence of the drug). This is, of course, the ideal. In real life it is impossible to conduct the proposed drug dosage experiment; the sheer number of possible independent variables in a group of potential patients would be high. Fortunately, while statisticians have come to the rescue in real life, you won’t need to understand the nuances of these statistical issues in BIS2A.
Accuracy in measurement, uncertainty, and replication
Finally, we mention the intuitive notion that the tools used to make the measurements in an experiment must be reasonably accurate. How accurate? They must be accurate enough to make measurements with sufficient certainty to draw conclusions about whether changes in independent variables actually influence the value of a dependent variable. If we take, yet again, the blood pressure example above. In that experiment, we made the important assumption that the experimenter had tools that allowed her to make accurate measurements of the changes in blood pressure associated with the effects of the drug. For instance if the changes associated with the drug ranged between 0 and 3 mmHg and her meter capably measured changes in blood pressure with a certainty of +/- 5 mmHg, she could not have made the necessary measurements to test her hypothesis or would have missed seeing the effect of the drug. For the sake of the example, we assume that she had a better instrument and that she could be confident that any changes she measured were indeed differences due to the drug treatment and that they were not due to measurement error, sample-to-sample variability, or other sources of variation that lower the confidence of the conclusions that are drawn from the experiment.
The topic of measurement error leads us to mention that there are numerous other possible sources of uncertainty in experimental data that you as students will ultimately need to learn about. These sources of error have a lot to do with determining how certain we are that experiments have disproven a hypothesis, how much we should trust the interpretation of the experimental results and, by extension, our current state of knowledge. Even at this stage, you will recognize some experimental strategies used to deal with these sources of uncertainty (i.e., making measurements on multiple samples, creating replicate experiments). You will learn more about this in your statistics courses later on.
For now, you should, however, be aware that experiments carry a certain degree of confidence in the results and that the degree of confidence in the results can be influenced by many factors. Developing healthy skepticism involves, among other things, learning to assess the quality of an experiment and the interpretation of the findings and learning to ask questions about things like this.
Note: possible discussion
After moving to California to attend UC Davis, you have fallen in love with fresh tomatoes. You decide that the tomatoes in the stores just don’t taste right and resolve to grow your own.
You plant tomato plants all over your back yard; every free space now has a freshly planted tomato seedling of the same variety. You have planted tomatoes in the ground in full sunlight and next to your house in full shade.
Observation: After the first year of harvest, you make the observation that the plants growing in full shade almost always seem shorter than those in the full sun. You think that you have a reasonable explanation (hypothesis) for this observation.
Based on the information above, you create the following hypothesis to explain the differences in height you noticed in your tomatoes:
Hypothesis: The height that my tomato plants reach is positively correlated to the amount of sunlight they are exposed to (e.g., the more sun the plant gets, the taller it will be).
This hypothesis is testable and falsifiable. So, the next summer you decide to test your hypothesis.
This hypothesis also allows you to make a prediction. In this case you might predict that IF you were to shade a set of tomatoes in the sunny part of the yard, THEN those plants would be shorter than their full-sun neighbors.
You design an experiment to test your hypothesis by buying the same variety of tomato that you planted the previous year and plant your whole yard again. This year, however, you decide to do two different things:
- You create a shade structure that you place over a small subset of plants in the sunny part of your yard.
- You build a contraption with mirrors that redirects some sunlight onto a small subset of plants that are in the shady part of the yard.
Question 1: We used a shortcut above. Can you create statements for both the null and alternative hypothesis? Work with your classmates to do this.
Question 2: Why do you create a shade structure? What is this testing? Based on your hypothesis what do you predict will happen to the plants under the shade structure?
Question 3: Why do you create the mirror contraption? Why do you potentially need this contraption if you already have the shade structure?
New data: At the end of the summer you measure the height of your tomato plants and you find, once again, that the plants in the sunny part of the yard are indeed taller than those in the shady part of the yard. However, you notice that there is no difference in height between the plants under your shade structure and those right next to the structure in full sun. In addition, you notice that the plants in the shady part of the yard are all about the same height, including those that had extra light shined on them via your mirror contraption.
Question 4: What does this experiment lead you to conclude? What would you try to do next?
Question 5: Imagine an alternative scenario in which you discovered, as before, that the plants in the sunny part of the yard were all the same height (even those under your shade structure) but that the plants in the shady part of the yard that got “extra” light from your mirror contraption grew taller than their immediate neighbors. What would this say about your alternate hypothesis? Null hypothesis? What would you do next?
Question 6: What assumptions are you making about the ability to make measurements in this experiment? What influence might these assumptions have on your interpretation of the results?
In this class, you will occasionally be asked to create a hypotheses, to interpret data, and to design experiments with proper controls. All of these skills take practice to master—we can start to practice them in BIS2A. Again, while we don’t expect you to be masters after reading this text, we will assume that you have read this text during the first week and that the associated concepts are not completely new to you. You can always return to this text as a resource to refresh yourself.
Disclaimer
While the preceding treatment of the experimental method is very basic—you will undoubtedly add numerous layer of sophistication to these basic ideas as you continue in your studies—it should serve as a sufficient introduction to the topic for BIS2A. The most important point to remember from this section is that the knowledge represented in this course, while sometimes inadvertently represented as irrefutable fact, is really just the most current hypothesis about how certain things happen in biology that has yet to be falsified via experiment.
The Design Challenge
Your BIS2A instructors have devised something that we call “The Design Challenge” to help us approach the topics we cover in the course from a problem solving and/or design perspective. This teaching tool helps us:
• develop a frame of mind or way of approaching the material and
• design a set of sequential steps that help structure thinking about course topics in a problem-solving context.
How is it intended to work? When we encounter a topic in class, “The Design Challenge” encourages us to think about it in the following problem-solving centric way:
- Identify the problem(s) - this may include identifying "big" problems and also decomposing them into "smaller" nested sub-problems
- Determine criteria for successful solutions
- Identify and/or imagine possible solutions
- Evaluate the proposed solutions against the criteria for success
- Choose a solution
By using the structure of the design challenge, topics that are typically presented as lists of facts and stories are transformed into puzzles or problems that need solving. For instance the discussion about the topic of cell division is motivated by a problem. The problem statement can be: "The cell needs to divide". Some of the criteria for success can include needing to have a near identical copy of DNA in each daughter cell, distributing organelles between the daughter cells so that each remains viable etc. These would be considered sub-problems to the larger “the cell needs to divide” problem. One can then go on to explore what the challenges are and try to use existing knowledge and imagination to propose some solutions for each of those problems. Different solutions can be evaluated and then compared to what Nature seems to have done (at least in the cases that are well studied).
This exercise requires us to use imagination and critical thinking. It also encourages the student and instructor to think critically about why the particular topic is important to study. The Design Challenge approach to teaching biology attempts to make the student and instructor focus on the important core questions that drove the development of the knowledge in the first place! It also encourages students to dream up new ideas and to interact with the material in a manner that is question/problem-centered rather than “fact”-centered. The question/problem-centered approach is different from what most people are used to, but it is ultimately more useful for developing skills, mental frameworks and knowledge that will transfer to other problems that they will encounter during their studies and beyond.
Example
For example, the guiding problem in BIS2A is to understand “How to Build a Cell”. This rather complex problem will be broken down into several smaller sub-problems that include:
- acquiring the building blocks to construct cellular parts from the environment
- acquiring the energy to build cellular parts from the environment
- transforming the building blocks of the cell between different forms
- transferring energy between different storage forms
- creating a new cell from an old cell
- problems we identify in class
As we explore these sub-problems, we will at times explore some of the different ways in which biology has addressed each issue. As we get into details, let us however make sure to stay focused on and not forget the importance of always staying linked to the questions/problems that motivated us to talk about the specifics in the first plac
Scientific Method vs. The Design Challenge
At this point you might be thinking: "What is the difference between the scientific method and the design challenge rubric and why do I need both?" It's not an uncommon question so let's see if we can clarify this now.
The design challenge and the scientific method are both processes that share similar qualities. The critical distinguishing feature, however, is the purpose behind each of the processes. The scientific method is a process used for eliminating possible answers to questions. A typical scenario where one might use the scientific method would involve someone making an observation, proposing multiple explanations, designing an experiment that might help eliminate one or more of the explanations, and reflecting on the result. By contrast the design process is used for creating solutions to problems. A typical scenario for the design challenge would start with a problem that needs solving, defining criteria for a successful resolution, devising multiple possible solutions that would meet the success criteria and either selecting a solution or reflecting on changes that might be made to the designs to meet success criteria. A key operational difference is that the design challenge requires that criteria for success be defined while the scientific method does not.
While both are similar, the differences are still real and we need to practice both processes. We'll assert that we use both processes in "real life" all of the time. A physician, for instance, will use both processes interactively as she forms hypotheses that try to determine what might be causing her patient's ailments. She will turn around and use the design process to build a course of treatment that meets certain success criteria. A scientist may be deep into hypothesis generation but he will eventually need to use a design process for building an experiment that will, within certain definable success criteria, help him answer a question.
Both processes, while similar, are important to use in different situations and we want to begin getting better at both.
Evolution and Natural Selection
Brief overview
Evolution and natural selection are core concepts in biology that are typically invoked to help explain the diversity of and relationships between life on Earth, both extant and extinct. Fortunately, in BIS2A, you need to understand and use only a few core ideas related to evolution and natural selection. We describe these below. You will expand your understanding and add details to these core concepts in BIS2B and BIS2C.
The first idea you need to grasp is that evolution can be simply defined as the development/change of something over time. In the automotive industry, the shapes and features of cars can be said to evolve (change in time). In fashion, it can be said that style evolves. In biology, life and, in particular, reproducing populations of organisms with different traits evolve.
The second thing to understand is that natural selection is a process by which nature filters organisms in a population. What is the filter? Here it becomes a little more complicated (but only a little). The simplest explanation is that the selective filter is just a combination of all living and nonliving factors in an environment, which influence how successfully an organism can reproduce. The factors that influence the ability of an organism to reproduce are known as selective pressures. A small but important complication is that these factors are not the same everywhere; they change in time and by location. Thus, the selective pressures that create the filter are constantly changing (sometimes rapidly, sometimes slowly), and organisms in the same reproducing population could experience different pressures at different times and in different locations.
The theory of evolution by natural selection puts these two ideas together; it stipulates that change in biology happens over time and that the variation in a population is constantly subjected to selection based on how differences in traits influence reproduction. But what are these characteristics or traits? What traits/features/functions can be subject to selection? The short answer is: just about anything associated with an organism for which variation exists in a population and for which this variation leads to a differential likelihood of generating offspring will probably be subject to filtering by natural selection. We also call these traits heritable phenotypes. Organisms in a population that have phenotypes, which enable them to pass the selective filter more efficiently than others, are said to have a selective advantage and/or greater fitness.
It is important to reiterate that while the phenotypes carried by individual organisms may be subject to selection, the process of evolution by natural selection both requires and acts on phenotypic variation within populations. If neither variation nor populations in which that variation can reside exist, there is no opportunity or need for selection. Everything is and stays the same.
Common misconceptions and a course specific note
Finally, we draw your attention to a critical point and common misconception among beginning students in biology. This misconception can arise when, for the sake of discussion, we decide to anthropomorphize nature by giving it an intellect. For example, we may try to build an example for evolution by natural selection by proposing that a surplus of a particular food exists in an environment and there is an organism close by that is starving. It would be correct to reason that if the organism could eat that food that this might give it a selective advantage over other organisms that cannot. If later we find an example of organisms that have the capability to eat that surplus food, it might be tempting to say that nature evolved to solve the problem the surplus food. The process of evolution by natural selection, however, happens randomly and without direction. That is, nature does NOT identify “problems” that are limiting fitness. Nature does NOT identify features that would make an organism more successful and then start creating diverse solutions that meet this need. The generation of variation is not guided. Variation happens and natural selection filters what works best. The observation that an organism exists that can eat the surplus food is not a reflection of nature actively solving a problem, but rather, a reflection of whatever processes that led to phenotypic variation in an ancestral population that created—among many other variants—a phenotype that increased fitness (possibly because the ancestral organisms were able to eat the surplus food).
This point of the preceding paragraph is particularly important to understand in the context of BIS2A because of the way we will be utilizing the Design Challenge to understand biology. While the Design Challenge is intended to help focus our attention on functions under selection and their relationship to determining fitness, it can be easy—if we aren’t attentive—to lapse into language that would suggest that nature purposefully designs solutions to solve specific problems. Always remember that we are looking retrospectively at what nature has selected and that we are attempting to understand why a specific phenotype may have been selected over many other possibilities. In doing so, we will be inferring or hypothesizing to the best of our ability (which is sometimes wrong) a sensible reason to explain why a phenotype might have provided a selective advantage. We are NOT saying that the phenotype evolved TO provide a specific selective advantage. The distinction between these two ideas may be subtle, but it is critical!
Note: possible discussion
What physical traits can you think of that give a selective advantage to certain species? Under what conditions would this trait grant those advantages? Under what conditions might that trait be a selective disadvantage?
Note: possible discussion
The great varieties of domesticated dog breeds from which we can choose for companionship are also the result of a process of evolution by selection. Likewise, the development of many very different looking crops—cabbage, brussel sprouts, kohlrabi, kale, broccoli and cauliflower—is also the result of evolution by selection. However, in these two cases the selection or filtering process is referred to artificial selection rather than natural selection. Discuss how artificial and natural selection are similar and different?
Note: possible discussion
How do environmental and political factors influence manufacturing processes such as automobile design? Fashion? Etc. What aspects are similar to the evolution of an organism, and what aspects are different?
Note: possible discussion
A related but slightly different misconception about evolution by natural selection is that this process leads to the creation of the most efficient solutions to problems. What is the problem with this notion?
General Approach to Biomolecule Types in BIS2A
Before you start
If necessary please review the Design Challenge module to review the Design Challenge rubric.
Some context and motivation
In BIS2A, we are concerned primarily with developing a functional understanding of a biological cell. In the context of a design problem, we might say that we want to solve the problem of building a cell. If we break this big task down into smaller problems, or alternatively, ask what types of things do we need to understand in order to do this, it would be reasonable to conclude that understanding what the cell is made of would be important. That said, it isn't sufficient to appreciate WHAT the cell is made of. We also need to understand the PROPERTIES of the materials that make up the cell. This requires us to dig into a little bit of chemistry—the science of the "stuff" (matter) that makes up the world we know.
This prospect of talking about molecular chemistry and thermodynamics makes some students of biology apprehensive. Hopefully, however, we will show that many of the vast number of biological processes that we care about arise directly from the chemical properties of the "stuff" that makes up life and that developing a functional understanding of some basic chemical concepts can be tremendously useful in thinking about how to solve problems in medicine, energy, and environment by attacking them at their core.
Importance of chemical composition
As a student in BIS2A, you will be asked to classify macromolecules into groups by looking at their chemical composition and, based on this composition, also infer some of the properties they might have. For example, carbohydrates typically have multiple hydroxyl groups. Hydroxyl groups are polar functional groups capable of forming hydrogen bonds. Therefore, some of the biologically relevant properties of various carbohydrates can be understood at some level by a balance between how they may tend to form hydrogen bonds with water, themselves or other molecules.
Linking structure to function
Each macromolecule plays a specific role in the overall functioning of a cell. The chemical properties and structure of a macromolecule will be directly related to its function. For example, the structure of a phospholipid can be broken down into two groups, a hydrophilic head group and a hydrophobic tail group. Each of these groups plays a role in not only the assembly of the cell membrane but also in the selectivity of substances that can/cannot cross the membrane.
The Structure of an atom

Figure 1. Atoms are the building blocks of molecules found in the universe—air, soil, water, rocks—and also the cells of all living organisms. In this model of an organic molecule, the atoms of carbon (black), hydrogen (white), nitrogen (blue), oxygen (red), and sulfur (yellow) are shown in proportional atomic size. The silver rods represent chemical bonds. (credit: modification of work by Christian Guthier)
An atom is the smallest unit of matter that retains all of the chemical properties of an element. Elements are forms of matter with specific chemical and physical properties that cannot be broken down into smaller substances by ordinary chemical reactions.
The chemistry discussed in BIS2A requires us to use a model for an atom. While there are more sophisticated models, the atomic model used in this course makes the simplifying assumption that the standard atom is composed of three subatomic particles, the proton, the neutron, and the electron. Protons and neutrons have a mass of approximately one atomic mass unit (a.m.u.). One atomic mass unit is approximately 1.660538921 x 10-27kg—roughly 1/12 of the mass of a carbon atom (see table below for more precise value). The mass of an electron is 0.000548597 a.m.u. or 9.1 x 10-31kg. Neutrons and protons reside at the center of the atom in a region call the nucleus while the electrons orbit around the nucleus in zones called orbitals, as illustrated below. The only exception to this description is the hydrogen (H) atom, which is composed of one proton and one electron with no neutrons. An atom is assigned an atomic number based on the number of protons in the nucleus. Neutral carbon (C), for instance has six neutrons, six protons, and six electrons. It has an atomic number of six and a mass of slightly more than 12 a.m.u.
Table 1. Charge, mass, and location of subatomic particles
| Protons, neutrons, and electrons | ||||
| Charge | Mass (a.m.u.) | Mass (kg) | Location | |
| Proton | +1 | ~1 | 1.6726 x 10-27 | nucleus |
| Neutron | 0 | ~1 | 1.6749 x 10-27 | nucleus |
| Electron | –1 | ~0 | 9.1094 x 10-31 | orbitals |
Table 1 reports the charge and location of three subatomic particles—the neutron, proton, and electron. Atomic mass unit = a.m.u. (a.k.a. dalton [Da])—this is defined as approximately one twelfth of the mass of a neutral carbon atom or 1.660538921 x 10−27 kg. This is roughly the mass of a proton or neutron.

Figure 2. Elements, such as helium depicted here, are made up of atoms. Atoms are made up of protons and neutrons located within the nucleus and electrons surrounding the nucleus in regions called orbitals. (Note: This figure depicts a Bohr model for an atom—we could use a new open source figure that depicts a more modern model for orbitals. If anyone finds one please forward it.)
Source:(https://commons.wikimedia.org/wiki/F...um_atom_QM.svg)
By User: Yzmo (Own work) [GFDL (http://www.gnu.org/copyleft/fdl.html) or CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0/)], via Wikimedia Commons
Relative sizes and distribution of elements
The typical atom has a radius of one to two angstroms (Å). 1Å = 1 x 10-10m. The typical nucleus has a radius of 1 x 10-5Å or 10,000 smaller than the radius of the whole atom. By analogy, a typical large exercise ball has a radius of 0.85m. If this were an atom, the nucleus would have a radius about 1/2 to 1/10 of your thinnest hair. All of that extra volume is occupied by the electrons in regions called orbitals. For an ideal atom, orbitals are probabilistically defined regions in space around the nucleus in which an electron can be expected to be found.
For additional basic information on atomic structure click here.
For additional basic information on orbitals here.
Video clips
For a review of atomic structure check out this Youtube video: atomic structure.
The properties of living and nonliving materials are determined to a large degree by the composition and organization of their constituent elements. Five elements are common to all living organisms: Oxygen (O), Carbon (C), Hydrogen (H), Phosphorous (P), and Nitrogen (N). Other elements like Sulfur (S), Calcium (Ca), Chloride (Cl), Sodium (Na), Iron (Fe), Cobalt (Co), Magnesium, Potassium (K), and several other trace elements are also necessary for life, but are typically found in far less abundance than the "top five" noted above. As a consequence, life's chemistry—and by extension the chemistry of relevance in BIS2A—largely focuses on common arrangements of and reactions between the "top five" core atoms of biology.

Figure 3. A table illustrating the abundance of elements in the human body. A pie chart illustrating the relationships in abundance between the four most common elements.
Credit: Data from Wikipedia (http://en.wikipedia.org/wiki/Abundan...mical_elements); chart created by Marc T. Facciotti
The Periodic Table
The different elements are organized and displayed in the periodic table. Devised by Russian chemist Dmitri Mendeleev (1834–1907) in 1869, the table groups elements that, due to some commonalities of their atomic structure, share certain chemical properties. The atomic structure of elements is responsible for their physical properties including whether they exist as gases, solids, or liquids under specific conditions and and their chemical reactivity, a term that refers to their ability to combine and to chemically bond with each other and other elements.
In the periodic table, shown below, the elements are organized and displayed according to their atomic number and are arranged in a series of rows and columns based on shared chemical and physical properties. In addition to providing the atomic number for each element, the periodic table also displays the element’s atomic mass. Looking at carbon, for example, its symbol (C) and name appear, as well as its atomic number of six (in the upper right-hand corner indicating the number of protons in the neutral nucleus) and its atomic mass of 12.11 (sum of the mass of electrons, protons, and neutrons).
Electronegativity
Molecules are collections of atoms that are associated with one another through bonds. It is reasonable to expect—and the case empirically—that different atoms will exhibit different physical properties, including abilities to interact with other atoms. One such property, the tendency of an atom to attract electrons, is described by the chemical concept and term, electronegativity. While several methods for measuring electronegativity have been developed, the one most commonly taught to biologists is the one created by Linus Pauling.
A description of how Pauling electronegativity can be calculated is beyond the scope of BIS2A. What is important to know, however, is that electronegativity values have been experimentally and/or theoretically determined for nearly all elements in the periodic table. The values are unitless and are reported relative to the standard reference, hydrogen, whose electronegativity is 2.20. The larger the electronegativity value, the greater tendency an atom has to attract electrons. Using this scale, the electronegativity of different atoms can be quantitatively compared. For instance, by using Table 1 below, you could report that oxygen atoms (O) are more electronegative than phosphorous atoms (P).
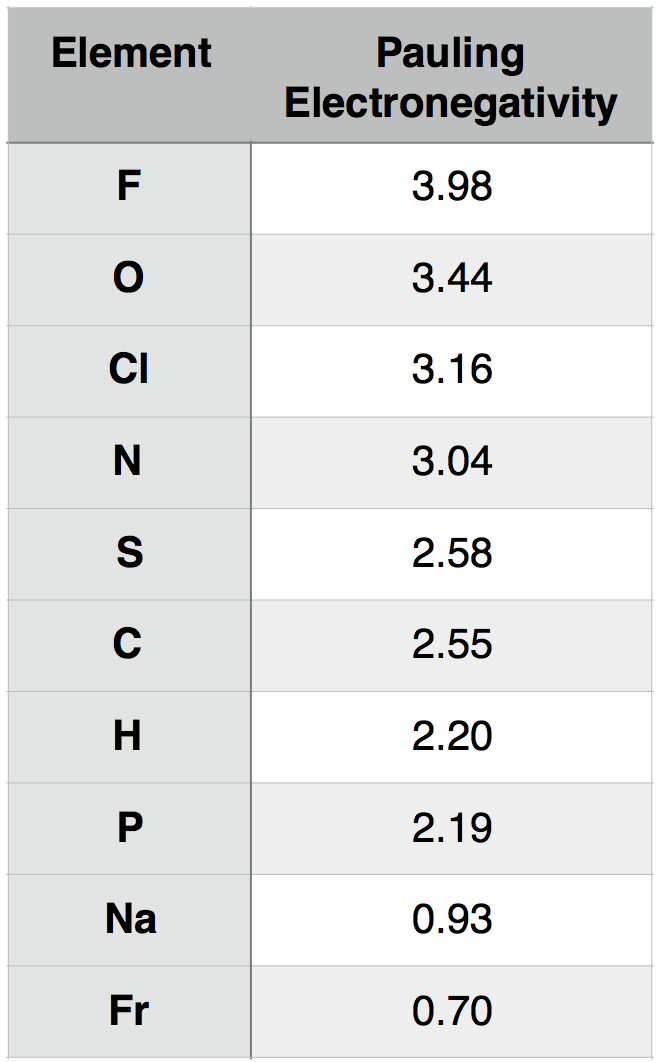
Table 1. Pauling electronegativity values for select elements of relevance to BIS2A as well as elements at the two extremes (highest and lowest) of the electronegativity scale.
Attribution: Marc T. Facciotti (original work)
The utility of the Pauling electronegativity scale in BIS2A is to provide a chemical basis for explaining the types of bonds that form between the commonly occurring elements in biological systems and to explain some of the key interactions that we observe routinely. We develop our understanding of electronegativity-based arguments about bonds and molecular interactions by comparing the electronegativities of two atoms. Recall, the larger the electronegativity, the stronger the "pull" an atom exerts on nearby electrons.
We can consider, for example, the common interaction between oxygen (O) and hydrogen (H). Let us assume that O and H are interacting (forming a bond) and write that interaction as O-H, where the dash between the letters represents the interaction between the two atoms. To understand this interaction better, we can compare the relative electronegativity of each atom. Examining the table above, we see that O has an electronegativity of 3.44, and H has an electronegativity of 2.20.
Based on the concept of electronegativity as we now understand it, we can surmise that the oxygen (O) atom will tend to "pull" the electrons away from the hydrogen (H) when they are interacting. This will give rise to a slight but significant negative charge around the O atom (due to the higher tendency of the electrons to be associated with the O atom). This also results in a slight positive charge around the H atom (due to the decrease in the probability of finding an electron nearby). Since the electrons are not distributed evenly between the two atoms AND, by consequence, the electric charge is also not evenly distributed, we describe this interaction or bond as polar. There are two poles in effect: the negative pole near the oxygen and the positive pole near the hydrogen.
To extend the utility of this concept, we can now ask how an interaction between oxygen (O) and hydrogen (H) differs from an interaction between sulfur (S) and hydrogen (H). That is, how does O-H differ from S-H? If we examine the table above, we see that the difference in electronegativity between O and H is 1.24 (3.44 - 2.20 = 1.24) and that the difference in electronegativity between S and H is 0.38 (2.58 – 2.20 = 0.38). We can therefore conclude that an O-H bond is more polar than an S-H bond. We will discuss the consequences of these differences in subsequent chapters.

Figure 2. The periodic table with the electronegativities of each atom listed.
Attribution: By DMacks (https://en.wikipedia.org/wiki/Electronegativity) [CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons
An examination of the periodic table of the elements (Figure 2) illustrates that electronegativity is related to some of the physical properties used to organize the elements into the table. Certain trends are apparent. For instance, those atoms with the largest electronegativity tend to reside in the upper right hand corner of the periodic table, such as Fluorine (F), Oxygen (O) and Chlorine (Cl), while elements with the smallest electronegativity tend to be found at the other end of the table, in the lower left, such as Francium (Fr), Cesium (Cs) and Radium (Ra).
More information on electronegativity can be found in the LibreTexts.
The main use of the concept of electronegativity in BIS2A will therefore be to provide a conceptual grounding for discussing the different types of chemical bonds that occur between atoms in nature. We will focus primarily on three types of bonds: Ionic Bonds, Covalent Bonds and Hydrogen Bonds.
Bond types
In BIS2A, we focus primarily on three different bond types: ionic bonds, covalent bonds, and hydrogen bonds. We expect students to be able to recognize each different bond type in molecular models. In addition, for commonly seen bonds in biology, we expect student to provide a chemical explanation, rooted in ideas like electronegativity, for how these bonds contribute to the chemistry of biological molecules.
Ionic bonds
Ionic bonds are electrostatic interactions formed between ions of opposite charges. For instance, most of us know that in sodium chloride (NaCl) positively charged sodium ions and negatively charged chloride ions associate via electrostatic (+ attracts -) interactions to make crystals of sodium chloride, or table salt, creating a crystalline molecule with zero net charge. The origins of these interactions may arise from the association of neutral atoms whose difference in electronegativities is sufficiently high. Take the example above. If we imagine that a neutral sodium atom and a neutral chlorine atom approach one another, it is possible that at close distances, due to the relatively large difference in electronegativity between the two atoms, that an electron from the neutral sodium atom is transferred to the neutral chlorine atom, resulting in a negatively charged chloride ion and a positively charged sodium ion. These ions can now interact via an ionic bond.

Figure 1. The formation of an ionic bond between sodium and chlorine is depicted. In panel A, a sufficient difference in electronegativity between sodium and chlorine induces the transfer of an electron from the sodium to the chlorine, forming two ions, as illustrated in panel B. In panel C, the two ions associate via an electrostatic interaction. Attribution: By BruceBlaus (own work) [CC BY-SA 4.0 (http://creativecommons.org/licenses/by-sa/4.0)], via Wikimedia Commons
This movement of electrons from one atom to another is referred to as electron transfer. In the example above, when sodium loses an electron, it now has 11 protons, 11 neutrons, and 10 electrons, leaving it with an overall charge of +1 (summing charges: 11 protons at +1 charge each and 10 electrons at -1 charge each = +1). Once charged, the sodium atom is referred to as a sodium ion. Likewise, based on its electronegativity, a neutral chlorine (Cl) atom tends to gain an electron to create an ion with 17 protons, 17 neutrons, and 18 electrons, giving it a net negative (–1) charge. It is now referred to as a chloride ion.
We can interpret the electron transfer above using the concept of electronegativity. Begin by comparing the electronegativities of sodium and chlorine by examining the periodic table of elements below. We see that chlorine is located in the upper-right corner of the table, while sodium is in the upper left. Comparing the electronegativity values of chlorine and sodium directly, we see that the chlorine atom is more electronegative than is sodium. The difference in the electronegativity of chlorine (3.16) and sodium (0.93) is 2.23 (using the scale in the table below). Given that we know an electron transfer will take place between these two elements, we can conclude that differences in electronegativities of ~2.2 are large enough to cause an electron to transfer between two atoms and that interactions between such elements are likely through ionic bonds.

Figure 2. The periodic table of the elements listing electronegativity values for each element. The elements sodium and chlorine are boxed with a teal boundary. Attribution: By DMacks (https://en.wikipedia.org/wiki/Electronegativity) [CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons—Modified by Marc T. Facciotti
Note: possible discussion
The atoms in a 5 in. x 5 in. brick of table salt (NaCl) sitting on your kitchen counter are held together almost entirely by ionic bonds. Based on that observation, how would you characterize the strength of ionic bonds?
Now consider that same brick of table salt after having been thrown into an average backyard swimming pool. After a couple of hours, the brick would be completely dissolved, and the sodium and chloride ions would be uniformly distributed throughout the pool. What might you conclude about the strength of ionic bonds from this observation?
Propose a reason why NaCl's ionic bonds in air might be behaving differently than those in water? What is the significance of this to biology?
For additional information:
Check out the link from the Khan Academy on ionic bonds.
Covalent bonds
We can also invoke the concept of electronegativity to help describe the interactions between atoms that have differences in electronegativity too small for the atoms to form an ionic bond. These types of interactions often result in a bond called a covalent bond. In these bonds, electrons are shared between two atoms—in contrast to an ionic interaction in which electrons remain on each atom of an ion or are transferred between species that have highly different electronegativities.
We start exploring the covalent bond by looking at an example where the difference in electronegativity is zero. Consider a very common interaction in biology, the interaction between two carbon atoms. In this case, each atom has the same electronegativity, 2.55; the difference in electronegativity is therefore zero. If we build our mental model of this interaction using the concept of electronegativity, we realize that each carbon atom in the carbon-carbon pair has the same tendency to "pull" electrons to it. In this case, when a bond is formed, neither of the two carbon atoms will tend to "pull" (a good anthropomorphism) electrons from the other. They will "share" (another anthropomorphism) the electrons equally, instead.
Aside: bounding example
The two examples above—(1) the interaction of sodium and chlorine, and (2) the interaction between two carbon atoms—frame a discussion by "bounding," or asymptotic analysis (see earlier reading). We examined what happens to a physical system when considering two extremes. In this case, the extremes were in electronegativity differences between interacting atoms. The interaction of sodium and chlorine illustrated what happens when two atoms have a large difference in electronegativities, and the carbon-carbon example illustrated what happens when that difference is zero. Once we create those mental goal posts describing what happens at the extremes, it is then easier to imagine what might happen in between—in this case, what happens when the difference in electronegativity is between 0 and 2.2. We do that next.
When the sharing of electrons between two covalently bonded atoms is nearly equal, we call these bonds nonpolar covalent bonds. If by contrast, the sharing of electrons is not equal between the two atoms (likely due to a difference in electronegativities between the atoms), we call these bonds polar covalent bonds.
In a polar covalent bond, the electrons are unequally shared by the atoms and are attracted to one nucleus more than to the other. Because of the unequal distribution of electrons between atoms in a polar covalent bond, a slightly positive (indicated by δ+) or slightly negative (indicated by δ–) charge develops at each pole of the bond. The slightly positive (δ+) charge will develop on the less electronegative atom, as electrons get pulled more towards the slightly more electronegative atom. A slightly negative (δ–) charge will develop on the more electronegative atom. Since there are two poles (the positive and negative poles), the bond is said to possess a dipole.
Examples of nonpolar covalent and polar covalent bonds in biologically relevant molecules
Nonpolar covalent bonds
Molecular oxygen
Molecular oxygen (O2) is made from an association between two atoms of oxygen. Since the two atoms share the same electronegativity, the bonds in molecular oxygen are nonpolar covalent.
Methane
Another example of a nonpolar covalent bond is the C-H bond found in the methane gas (CH4). Unlike the case of molecular oxygen where the two bonded atoms share the same electronegativity, carbon and hydrogen do not have the same electronegativity; C = 2.55 and H = 2.20—the difference in electronegativity is 0.35.

Figure 3. Molecular line drawings of molecular oxygen, methane, and carbon dioxide. Attribution: Marc T. Facciotti (own work)
Some of you may now be confused. If there is a difference in electronegativity between the two atoms, is the bond not by definition polar? The answer is both yes and no and depends on the definition of polar that the speaker/writer is using. Since this is an example of how taking shortcuts in the use of specific vocabulary can sometimes lead to confusion, we take a moment to discuss this here. See the mock exchange between a student and an instructor below for clarification:
1. Instructor: "In biology, we often say that the C-H bond is nonpolar."
2. Student: "But there is an electronegativity difference between C and H, so it would appear that C should have a slightly stronger tendency to attract electrons. This electronegativity difference should create a small, negative charge around the carbon and a small, positive charge around the hydrogen."
3. Student: "Since there is a differential distribution of charge across the bond, it would seem that, by definition, this should be considered a polar bond."
4. Instructor: "In fact, the bond does have some small polar character."
5. Student: "So, then it's polar? I'm confused."
6. Instructor: "It has some small amount of polar character, but it turns out that for most of the common chemistry that we will encounter that this small amount of polar character is insufficient to lead to "interesting" chemistry. So, while the bond is, strictly speaking, slightly polar, from a practical standpoint it is effectively nonpolar. We therefore call it nonpolar."
7. Student: "That's needlessly confusing; how am I supposed to know when you mean strictly 100% nonpolar, slightly polar, or functionally polar when you use the same word to describe two of those three things?"
8. Instructor: "Yup, it sucks. The fix is that I need to be as clear as I can when I talk with you about how I am using the term "polarity." I also need to inform you that you will find this shortcut (and others) used when you go out into the field, and I encourage you to start learning to recognize what is intended by the context of the conversation.
A real-world analogy of this same problem might be the use of the word "newspaper". It can be used in a sentence to refer to the company that publishes some news, OR it can refer to the actual item that the company produces. In this case, the disambiguation is easily made by native English speakers, as they can determine the correct meaning from the context; non-native speakers may be more confused. Don't worry; as you see more examples of technical word use in science, you'll learn to read correct meanings from contexts too."
Aside:
How large should the difference in electronegativity be in order to create a bond that is "polar enough" that we decide to call it polar in biology? Of course, the exact value depends on a number of factors, but as a loose rule of thumb, we sometimes use a difference of 0.4 as a guesstimate.
This extra information is purely for your information. You will not be asked to assign polarity based on this criteria in BIS2A. You should, however, appreciate the concept of how polarity can be determined by using the concept of electronegativity. You should also appreciate the functional consequences of polarity (more on this in other sections) and the nuances associated with these terms (such as those in the discussion above).
Polar covalent bonds
The polar covalent bond can be illustrated by examining the association between O and H in water (H2O). Oxygen has an electronegativity of 3.44, while hydrogen has an electronegativity of 2.20. The difference in electronegativity is 1.24. It turns out that this size of electronegativity difference is large enough that the dipole across the molecule contributes to chemical phenomenon of interest.
This is a good point to mention another common source of student confusion regarding the use of the term polar. Water has polar bonds. This statement refers specifically to the individual O-H bonds. Each of these bonds has a dipole. However, students will also hear that water is a polar molecule. This is also true. This latter statement is referring to the fact that the sum of the two bond dipoles creates a dipole across the whole molecule. A molecule may be nonpolar but still have some polar bonds.

Figure 4. A water molecule has two polar O-H bonds. Since the distribution of charge in the molecule is asymmetric (due to the number and relative orientations of the bond dipoles), the molecule is also polar. The element name and electronegativities are reported in the respective sphere. Attribution: Marc T. Facciotti (own work)
For additional information, view this short video to see an animation of ionic and covalent bonding.
The continuum of bonds between covalent and ionic
The discussion of bond types above highlights that in nature you will see bonds on a continuum from completely nonpolar covalent to purely ionic, depending on the atoms that are interacting. As you proceed through your studies, you will further discover that in larger, multi-atom molecules, the localization of electrons around an atom is also influenced by multiple factors. For instance, other atoms that are also bonded nearby will exert an influence on the electron distribution around a nucleus in a way that is not easily accounted for by invoking simple arguments of pairwise comparisons of electronegativity. Local electrostatic fields produced by other non-bonded atoms may also have an influence. Reality is always more complicated than are our models. However, if the models allow us to reason and predict with "good enough" precision or to understand some key underlying concepts that can be extended later, they are quite useful.
Key bonds in BIS2A
In BIS2A, we are concerned with the chemical behavior of and bonds between atoms in biomolecules. Fortunately, biological systems are composed of a relatively small number of common elements (e.g., C, H, N, O, P, S, etc.) and some key ions (e.g., Na+, Cl-, Ca2+, K+, etc.). Start recognizing commonly occurring bonds and the chemical properties that we often see them showing. Some common bonds include C-C, C-O, C-H, N-H, C=O, C-N, P-O, O-H, S-H, and some variants. These will be discussed further in the context of functional groups. The task is not as daunting as it seems.








