1.7: Aseptic Technique
- Page ID
- 79233
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Differentiate between the types of microbiological media.
- Define aseptic, aseptic technique, pure culture, contamination, sterilization, autoclave, disinfectant, and antiseptic.
- Successfully use aseptic technique in microbiology transfers.
- Describe good aseptic technique in microbiology transfers.
- Recognize examples of good and bad aseptic technique and possible sources of contamination.
Microbiological Media
To study bacteria and other microorganisms, it is necessary to grow them in controlled conditions. Microbes are grown in substances that provide the nutrients necessary to sustain their metabolic activities and reproduction called "growth media" or simply "media" (singular is "medium"). Growth media can be either liquid or solid.
A liquid medium is called a broth. Broths can be used to determine growth patterns in a liquid medium, and for certain types of inoculations and metabolic tests. They are also the method of choice for growing large quantities of bacteria.
Solid growth media usually contains agar, which is a mixture of polysaccharides derived from red algae. It is used as a solidification agent because it (1) is not broken down by bacteria, (2) contains no nutrients that can be used by bacteria and (3) melts at high temperatures, and yet is solid at temperatures used for most bacterial growth. Solid growth media is used in the following forms: agar plates, agar slants and agar deeps. Making solid media is similar to making Jell-O, where a powder is mixed into water and heated to fully dissolve the powder. When the solution cools it solidifies. Melted agar is poured into a test tube and then allowed to solidify vertically for an agar deep, or at an angle for an agar slant. Agar plates are made by pouring melted agar into a petri dish. (Petersen, 2016)
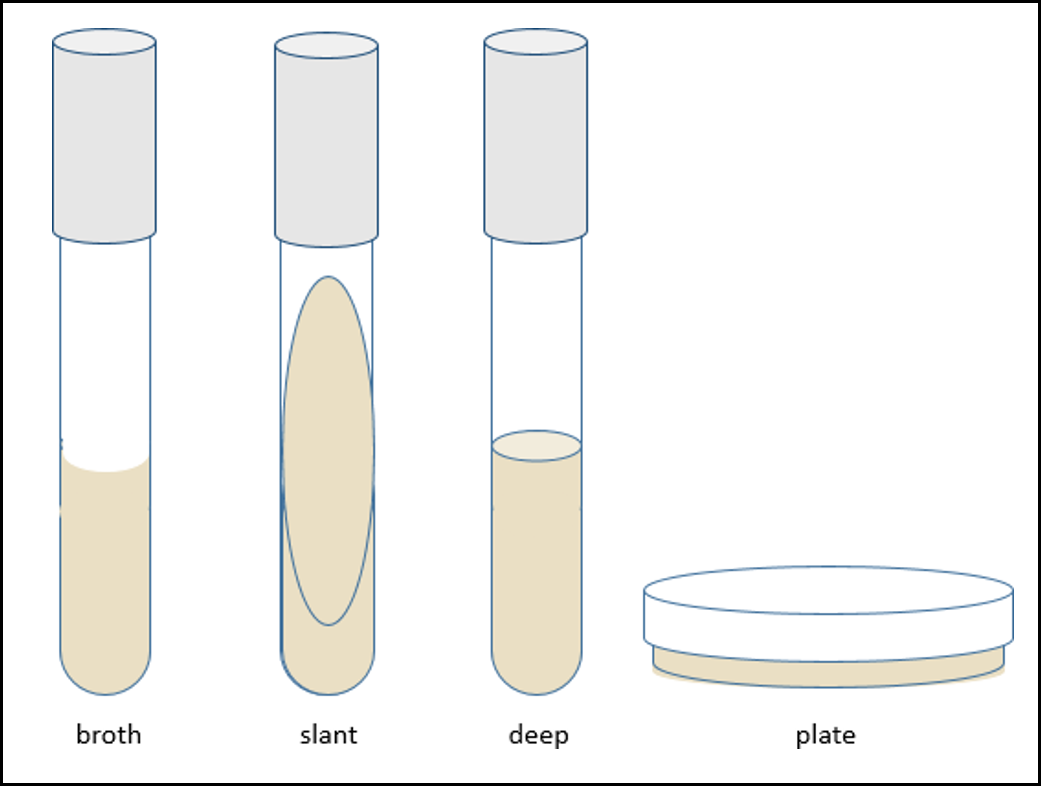
Figure 1: Illustration showing the different forms microbiological media can be prepared as.
Because of the relatively small tube opening (less opportunity to dry out or become contaminated) and the surface area available for growth, agar slants are commonly used to culture and store bacteria for intermediate periods of time (weeks). These types of cultures are called stocks. Deeps are often used to for certain differential metabolic tests.
In contrast to deeps and slants, agar plates have a large surface area for growth. Bacterial cells can be spread out over the surface so that they form discrete colonies which can be characterized. In a few weeks, you will be using a series of plate cultures to separate two different microbes from a mixture. In addition, specialized media in plate form is used for certain biochemical tests. (Petersen, 2016)
Media Contamination
Microbiologists generally study the organisms in pure culture, a culture that contains a single microbial species. If an unintended microorganism is introduced into a pure culture, the culture becomes contaminated. Aseptic technique is the collection of procedures and techniques designed to prevent the introduction of unwanted organisms into a pure culture or into the laboratory environment.
The term “aseptic” literally means “without contamination.” These procedures are as important for the experimenter’s safety as they are for maintaining culture purity.
Sterilization is the complete removal all vegetative cells, endospores, and viruses from an item (OpenStax CNX, 2018). Sterilization is all or none; something is either sterile or it is not sterile. In this course, all media, the substance in which the cells are grown, is sterilized by autoclave.
An autoclave uses moist heat (steam) under pressure to destroy all life forms. Whereas most vegetative cells can be killed at temperatures between 60 and 80oC, bacterial spores require temperatures above boiling (>100oC) for destruction. With a pressure of 15-20 lbs./in2, the autoclave can achieve a temperature of 121-132oC. Media under these conditions for at least 20 minutes will kill all spores as well as vegetative cells. Larger volumes require longer exposure times to ensure sufficient heat transfer to the materials being sterilized. The steam must directly contact the liquids or dry materials being sterilized, so containers are left loosely closed and instruments are loosely wrapped in paper or foil. The key to autoclaving is achieving a temperature high enough to kill spores for complete sterilization (OpenStax CNX, 2018).
Disinfection is the killing or growth inhibition of vegetative microbes. Generally, spores and some hearty cells will survive disinfection. Chemical disinfectants, such as chlorine bleach or products containing chlorine, are used to clean nonliving surfaces such as laboratory benches, clinical surfaces, and bathroom sinks (OpenStax CNX, 2018). We will use a chorine-based disinfectant to clean our work surfaces and to clean up any culture spills. Note that sterilization and disinfection are not interchangeable! (Why?) Spraying your bench top with disinfectant does not make it sterile.
Antiseptics are antimicrobial chemicals safe for use on living skin or tissues. Examples include hydrogen peroxide and isopropyl alcohol (OpenStax CNX, 2018).
When working in a microbiology laboratory, you must always remember that bacteria are present on all surfaces in the lab, as well as on your own hands and clothing. Aseptic techniques are designed to prevent the transfer of bacteria from the surrounding environment into a culture medium and from a culture to the environment. These techniques require care, concentration and practice. (Petersen, 2016)
Transfer Procedures
Because these procedures are completely new to most students, I strongly recommend that you watch the video at least twice. Keep in mind the following principles. (Some of these have been covered in the Laboratory Safety Exercise. They bear repeating because they are very important to keep you safe.)
Always begin by preparing your work area and making the necessary labels. Make sure you are clear about what transfers need to be made. The incinerator should be turned on HI for at least 20 minutes prior to using.
A transfer can be thought of in two parts, obtaining the cells (inoculum) from the source/parent culture and inoculating the new sterile tube or plate. Transfers, with very few exceptions, are performed by a single individual. You should not be holding the tube while your partner inoculates it.
Tutorial of Basic Aseptic Technique (slant to slant)
Before you Start
- Culture media must initially be sterile. Inspect your media before you start. If a culture medium appears cloudy or you observe unwanted growth, consult with your TA or instructor to be sure it is not contaminated before using it.
- Label your tubes white label tape. Masking tape is provided for other uses.
- Label plates on the bottom.
- Inspect the parent cultures. If the cells have fallen to the bottom, be sure to re-suspend them by flicking the tube gently to mix. Never shake a tube.
- Disinfect the lab bench.
- Light the Bunsen burner. The flame show be blue and a moderate height (not to tall, not too short).
Sterilizing the Inoculating Loop or Needle
- Hold the inoculating loop in your dominant hand like a pencil. To sterilize, place it in the Bunsen burner for at least 10 seconds. The entire wire must be heated red hot. Use the center blue region of the flame (not the top or bottom of the flame). Watch the clock for the time. Students tend to count too fast.
- Do not let the loop sit in the incinerator more than 15 seconds.
- Hold the instrument in the air allowing the wire to cool for about 15 seconds before making any transfers. Please do not wave it around to cool it.
- The wire is now sterile. If at this time, you set it down on the bench top, which is not sterile, it must be incinerated again before going into any culture. If a sterile instrument is touched to anything not sterile including your hand, sleeve, the outside of a tube or plate, a slide or the bench top, it becomes contaminated and cannot be used in an aseptic transfer.

Figure 2: How to hold an inoculation loop or needle for left-handed people (L) and for right-handed people.
Obtaining the Inoculum from a Tube Culture
- With your non-dominate hand, pick up the parent tube by grasping the tube just below the cap and lifting it out of the rack.
- Grasp the cap with the pinky and ring finger of your dominate hand and gently twist the tube out of the cap keeping your dominate hand still. See Figures 3. The cap is kept in your hand and never placed on the bench top.
- Heat the mouth of the open tube by passing it through the flame of the Bunsen burner. Heating creates convection currents, which carry airborne particles away from the mouth of the tube, preventing contamination of the culture or medium within.
- For a broth parent culture: Place the cooled loop into the broth and remove making sure that you have a thin film of liquid filling the loop. Jiggling the loop in the broth is not needed and can result in the formation of tiny aerosol droplets. Please do not jiggle the wire.
- For a slant parent culture: Touch the cooled loop to the growth. Do not break the agar surface. Refrain from “swiping” a large mass of cells. You do not need to see cells on the loop to have millions!
- Again, heat the mouth of the tube after withdrawing the transfer instrument. This step incinerates any microbes that may have been deposited on the lip of the tube during the transfer.
- Replace the cap and set the parent tube back in the test tube rack.
- Keep the inoculating instrument in your hand.

Figure 3: Grasping, removing, and hold a test tube cap while holding an inoculation loop or needle. The cap should never be placed on the bench top and the open end of the cap should not tough anything to avoid contamination.
To obtain the inoculum from a plate culture
- Turn the culture plate with bacteria growing on it right side up.
- Lift the lid a short distance, with your non-dominate hand, so that the lid acts at a shield protecting the agar surface from falling microbes in the air. See Figure 4.
- Touch the cooled loop to the growth. Do not breath the agar surface. Refrain from “swiping” a large mass of cells. You do not need to see cells on the loop to have millions!
- Replace the lid immediately after withdrawing the transfer instrument and turn the plate upside-down again.
- Keep the inoculating instrument in your hand.

Figure 4: Whenever transferring bacteria to or from a petri plate, the lid should only be lifted just enough to allow the loop to reach the agar. The lid of the petri plate should never be set down on the bench and should never touch anything to avoid contamination.
Inoculating a slant
- With your non-dominate hand, pick up the parent tube by grasping the tube just below the cap and lifting it out of the rack.
- Grasp the cap with the pinky and ring finger of your dominate hand and gently twist the tube out of the cap. Keeping your dominate hand still is especially important because there are cells on the loop at this point. Keep the cap in your hand.
- Pass the mouth of the tube through the flame.
- Insert the loop all the way to the bottom of the slant surface.
- Drag the loop on the agar “snaking” your way up the slant creating a “fishtail pattern.” This is called a fishtail inoculation. See Figure 5.
- Again, heat the mouth of the tube after withdrawing the transfer instrument. Replace the cap and set the parent tube back in the test tube rack.
- Immediately flame the inoculating loop and wire for a full 10 seconds before setting it down.

Figure 5: Inoculating a slant. Begin with the loop at the bottom of the slant you are transferring bacteria to and snake the loop up the surface of the slant.
Inoculating a broth
- With your non-dominate hand, pick up the parent tube by grasping the tube just below the cap and lifting it out of the rack.
- Grasp the cap with the pinky and ring finger of your dominate hand and gently twist the tube out of the cap. Keeping your dominate hand still is especially important because there are cells on the loop at this point.
- Pass the mouth of the tube through the flame.
- Insert the loop to the bottom of the broth liquid and then remove the loop. Jiggling is not necessary to dislodge cells.
- Again, heat the mouth of the tube after withdrawing the transfer instrument. Replace the cap and set the parent tube back in the test tube rack.
- Immediately flame the inoculating loop and wire for a full 10 seconds before setting it down.
After all inoculations
- Cultures to be incubated should be placed in the designated area for culture incubation. Otherwise, a student’s culture may be disposed of accidentally.
- Be sure to turn it off the Bunsen burner when you are finished with it.
Because there is so much to remember, the first time you make transfers many of the above actions are repeated in context. After a few weeks practice, the repetition will no longer be necessary and it will be assumed that you will adhere to the procedures above without reminder.
Practice Aseptic Technique
- Aseptically transfer a loop of sterile TSB to another test tube of sterile TSB:
- Flame loop.
- Remove cap from one test tube of sterile TSB and hold it in your hand (don't put it down and don't touch the open end).
- Pass the mouth of the test tube through the flame.
- Insert the flamed loop into the sterile TSB.
- There should be a film of liquid across the loop (similar to how a bubble wand will have a film across it).
- Pull the loop out of the test tube.
- Pass the mouth of the test tube through the flame.
- Put the cap back onto the test tube.
- Remove cap from the other test tube of sterile TSB and hold it in your hand (don't put it down and don't touch the open end).
- Pass the mouth of the test tube through the flame.
- Insert the loop with the film of sterile TSB.
- Pass the mouth of the test tube through the flame.
- Put the cap back onto the test tube.
- Flame the loop.
- Let each person in your group repeat with the same two test tubes.
- If every member of the group successfully transferred the TSB aseptically, there will be no growth (no turbidity) next class.
- Put labels on both culture tubes with your name and “aseptic.”
- Check the culture tubes next class for turbidity to determine whether or not your aseptic transfer was successful. A successful transfer would result in both tubes being clear (no growth).
Aseptic Technique Results
- Record your results in the table below.
|
Growth (+) or No Growth (-) |
|
|---|---|
|
TSB culture tube 1 |
|
|
TSB culture tube 2 |
- If you observed growth in the TSB culture tubes, what might have gone wrong? If you were successful in keeping both sterile, what are some possible sources of error that could cause contamination?
- Explain the importance of aseptic technique in microbiology.
- What is the purpose of flaming in aseptic technique?
- What are some signs of growth in a liquid medium?
Works Cited
- Petersen, J. a. (2016). Laboratory Excercises in Microbiology: Discovering the Unseen World Through Hands-On Investigation. CUNY Academic Works. Retrieved from http://academicworks.cuny.edu/qb_oers/16
Attributions
- Chapter Image: FISHER BUNSEN (6138440356).jpg by Jason Woodhead is licsenced under CC BY 2.0
- Klamm’s Microbiology Laboratory Manual by Loretta Sanderson Klamm is licensed under CC BY-NC-SA 4.0
- OpenStax CNX. (2018, Mar 19). OpenStax Microbiology, Microbiology . Retrieved from http://cnx.org/contents/e42bd376-624b-4c0f-972f-e0c57998e765@4.24


