Winter_2024_Bis2A_Facciotti_Slides_Reading_22_23
- Page ID
- 132602
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
| LECTURE SLIDES AND TRANSCRIPT | CORRESPONDING TEXTBOOK SECTION | ||||||||||||||||||||||||||||||
|
I want to start this next little bit by contemplating four ideas and then see how they're interrelated. |
Not covered in textbook |
||||||||||||||||||||||||||||||
|
I made a Terminator reference a couple of lectures ago. Here's one from Ratatouille. It's not quite as old of a movie. So, the grumpy old restaurant critic has one of my favorite lines in the movie. Everyone is nervous about the critic and he asks not for a specific dish, but he asks for perspective. “Hit me with your best shot.” What kind of perspective do we want to have here? |
Not covered in textbook | ||||||||||||||||||||||||||||||
|
One bit of perspective can come from a little drawing I made a couple of years ago. The point of this is that the cell is a, is a complicated place, right? We've learned about a small amount of biology this quarter. But nevertheless, if you try to represent even what we've learned this quarter in a picture, you can already see there's a lot of moving and interconnected parts. There's a lot of things that are talking to one another, different processes, et cetera. So, idea number one is that the cell is a complex and interconnected place. |
Not covered in textbook | ||||||||||||||||||||||||||||||
|
The second idea is kind of more familiar or real life. It is this idea of switches. We see these types of things all the time. |
Not covered in textbook | ||||||||||||||||||||||||||||||
|
Here's a light switch. What is its function? Turn on the light, turn off the light, right? Why do we want to turn on the light and turn off the light? Whose parents said, every time I walked by the bathroom, “turn off the light!” I have to do that all the time now at home. My parents used to do that to me too. Why do we want to turn on and off the lights? What are we managing? We’re managing energy, right? Parents are telling their kids to turn off the lights because they are concerned about the energy and the money that's going wasted when the lights on and nobody is in the room. Alright, we use these things to manage energy. To the idea of whether a switch is modular or not. Does anybody have a sense of whether a light switch like this, one you can find in the hardware store, is considered a modular thing? Can you take one that might be sitting in your bedroom and wire it a different room to control the lights there? Can replace the switch itself with a different switch? Yeah, so yeah, I would, I would call these type of things modular things, right? They're, a unit that has a certain function that can be moved around to different places and still carry out that function. You can still use the switch to turn on and off either some hobby project or replace it with a different one that looks different but has the same function. Either way it enables the choice of managing the energy. |
Not covered in textbook | ||||||||||||||||||||||||||||||
|
Here's some other switches that we might be familiar with. They are not binary switches like that one I just showed you, where it's either on or off. But you got switches in your, on your stove that allow you to kind of manage, not just in a binary way, but kind of in a continuous way the amount of energy that you're using on each of your burners. As you're cooking, whether it's an electric stove or gas stove, you're either cranking it super high or it's medium or low or anywhere in between. And then there are other switches that you might have around your house that have some sensors built into them. We've got some smart switches that can sense the environment and make some decision about, in this case, again, managing energy in your house. Whether you're running your air conditioner or your furnace, you can set each to run based on some sort of environmental variable. In this case, the temperature in the room. Here's a switch from a washing machine, where the switch can actually have a number of different functions. And depending on how it's used, it can trigger different discrete outcomes, right? So we can make, not just simple on-off or graded decisions. We got switches that allow us to choose between a variety of different programs to run. Alright. So that's idea number two. So, we have cells: a complicated place. Switches are really good for, for managing energy. And we have different types of switches. We got binary ones which are graded ones. We've got sensory switches, et cetera. |
Not covered in textbook | ||||||||||||||||||||||||||||||
|
Another thing idea here is that the statement that each step in the central dogma is a good place to make a decision. |
Not covered in textbook | ||||||||||||||||||||||||||||||
|
So, we've got the central dogma here? How do we decide whether to replicate DNA or to transcribe DNA? If we're going down this route, transcription, how do we decide which genes to transcribe? How do we decide which genes to translate? How do we decide which genes to degrade, etc. Each one of these arrows in our figure is an opportunity for some kind of control. And I'm going to stipulate that it's controlling resources or energy in the cell. Right? So, you're controlling the use of the raw materials that you've worked hard to harvest from your food and then likely taken through some metabolism. It takes a lot of nucleotides to make DNA. It takes a lot of nucleotides to make RNA. It takes a lot of energy to make protein. Remember, when we were looking at a cell’s dry mass. Most of the dry mass of the cell was made out of protein. There's a lot of energy invested into making protein. A lot of ATP and GTP burned in both cases. A lot of amino acids. So, each one of these steps of the central dogma is an important place to maybe put a switch of some sort on; to manage the energy and raw materials that we've been talking about all quarter. |
Introduction to gene regulationRegulation is all about decision making. Gene regulation is, therefore, all about understanding how cells decide about which genes to turn on, turn off or tune up or tune down. In the following section, we discuss some fundamental mechanisms and principles used by cells to regulate gene expression in response to changes in cellular or external factors. This biology is important for understanding how cells adjust changing environments, including how some cells, in multicellular organisms, become specialized for certain functions (e.g. tissues). Gene ExpressionIntroduction All cells control when and how much each one of its genes is expressed. This simple statement - one that could be derived from observing cellular behavior - brings up many questions that we can decompose using our Design Challenge rubric. Trying to define "gene expression"The first thing we need to do, however, is to define what it means when we say that a gene is "expressed". If the gene encodes a protein, one might reasonably propose that "expression" of a gene means how much functional protein the cell makes. But what if the gene does not encode a protein but some functional RNA. Then, in this case, "expressed might mean how much of the functional RNA the cell makes. Yet another person might reasonably suggest that "expression" just refers to the initial step in creating a copy of the genomic information. By that definition, one might want to count how many full-length transcripts are being made. Is it the number of end products encoded by the genomic information or is it the number of reads of the information important to describe "expression" properly. Unfortunately, in practice, we often find that the definition depends on the context of the discussion. Keep that in mind. For the sake of making sure that we are talking about the same thing, in Bis2A we'll try to use the term "expression" primarily to describe the creation of the final functional product(s). Depending on the specific case, the final product may be a protein or RNA species. |
||||||||||||||||||||||||||||||
|
Then the last thing is a little bit unrelated, but brings us to the rest of the lecture, which is, we can go back to weeks 1 and 2 and talk about this idea that proteins can interact with DNA. |
Not covered in textbook | ||||||||||||||||||||||||||||||
|
And those interactions are gonna be based on the ability of functional groups that are hanging off the amino acid side chains to interact with the phosphate backbone or to form specific hydrogen bonds with nucleotide bases in the DNA. So those lessons from again, week, week one, week two, we still want to keep in mind. Alright, and in this case actually, I'll add that we have alpha helices. We should be able to look at this picture and say that an alpha helix here, it's a little bit much for you guys right now to say, but the alpha helix is intercalating in the major groove. Remember the major groove? And there are hydrogen bonds formed between functional groups and amino acids and nucleotides. Alright, so. |
Possible NB Discussion What types of interactions do you think happen between the amino acids of the transcription factor and the double helix of the DNA? How do transcription factors recognize their binding site on the DNA? |
||||||||||||||||||||||||||||||
|
That is to set up this idea that there's a problem we want to solve, which is the regulation of transcription and translation. Let's start with transcription. I'll state the transcription needs to be regulated. So why, why would we assert that? Transcription uses energy, right? So if you transcribe every gene in the genome and you don't need 90% of them you are wasting 90% of the raw materials you brought in and the energy you extracted. So, we need to regulate transcription to manage our energy and raw materials. And we also need to manage transcription because it might be considered step one in the cell for deciding how it's going to respond to things that it sees into the environment. And if you’re a little microbe, the environment can be the pH of the environment, it can be the temperature, it can be the light that's in the environment if you're photosynthetic. Or if you don't like UV radiation, you might want to adjust your position in your environment to get away from UV. If you're a cell in our bodies, for instance, the environment might be what is floating around and what I get from the bloodstream that's feeding me. Am I getting signals from other organs that I need to respond to somehow? It could be the cells that are your neighbors in a tissue saying, “Hey, we there's a threat here, we need to respond to it somehow.” So, regulating transcription is important, again for this energy and raw materials management, but also because it's can be the first step in driving which genes are going to be expressed in response to changes around and in us Alright, what are some sub-problems to regulating transcription. What do I need to do if I need to regulate transcription, What's the first problem I need to solve? Yeah, knowing when and where to start. Also, knowing when to stop. When and where are important here. What else? What else did we talk about when we talked about regulating metabolic pathways earlier? It's basically the who, what, why, where, when, etc, right? Alright, so alright, when, why, how, where, all those questions. Alright, so these are some of the things that we want to answer today. If we had to like to stipulate some conditions for success of all these mechanisms that we might learn about. What makes a good solution for regulating transcription? How does nature decide what a good solution is? I heard mumble over here. Natural selection, right. And, what does natural selection tell us when it says the solution is good? It's letting an organism live. It's giving it some sort of selective advantage over other members in the population. Okay, Good. Let's talk about these questions. The who, what, why, where, and how of regulating transcription. |
The design challenge of regulating gene expressionTo drive this discussion from a design challenge perspective, we can formally stipulate that the "big problem" we are interested in understating is that of regulating protein abundance in a cell. Problem: The cell must regulate the abundance of each functional protein. We can then start by posing subproblems:
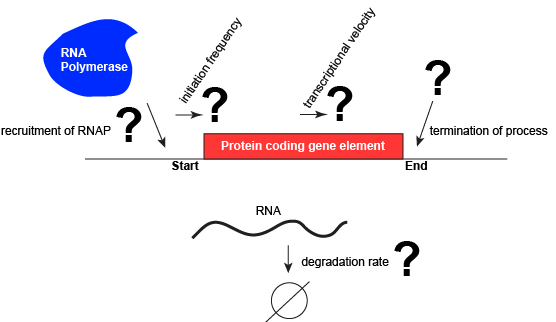
Focusing on transcriptionIn this course we begin by focusing primarily on examining the first couple of problems/questions, regulating transcription initiation and termination - from genomic information to a functional RNA, either ready as is (e.g. with a functional RNA) or ready for translation. This allows us to examine some fundamental concepts regarding the regulation of gene expression and to examine a few real examples of those concepts in action. Subproblems for transcription and the activity or RNA polymeraseLet us consider a protein-coding gene and work through some logic. We start by imagining a simple case, where a protein-coding gene is encoded by a single contiguous stretch of DNA. We know that to transcribe this gene an RNA polymerase will need to be recruited to the start of the coding region. The RNA polymerase is not "smart" per se. There needs to be some mechanism, based on chemical logic, to help recruit the RNA polymerase to the start of the protein-coding gene. Likewise, if this process is to be regulated, there needs to be some mechanism, or mechanisms to dictate when an RNA polymerase should be recruited to the start of a gene, when it should not, and/or if it is recruited to the DNA whether it should begin transcription and how many times this process should happen. Note, that the previous sentence, has several distinct subproblems/questions (e.g. when is the polymerase recruited?; if recruited, should it start transcription?; if it starts transcription, how many times should this process repeat?). We can also reasonably infer, that there will need to be some mechanisms to "instruct" (more anthropomorphisms) the polymerase to stop transcription. Finally, since the role of transcription is to create RNA copies of the genome segments, we should also consider problems/questions related to other factors that influence the abundance of RNA, like mechanisms of degradation. There must be some mechanisms, and these mechanisms will probably involve regulating this process.
A schematic showing a protein-coding gene and some questions or problems that we need to ask ourselves or problems we need to know solutions for if we are to understand how regulation of the transcriptional portion of the gene's expression is regulated. Attribution: Marc T. Facciotti (own work) Activation and Repression of TranscriptionSome basicsLet us consider a protein-coding gene and work through some logic. We start by imagining a simple case, where a protein-coding gene is encoded by a single contiguous stretch of DNA. We know that to transcribe this gene an RNA polymerase will need to be recruited to the start of the coding region. The RNA polymerase is not "smart" per se. There needs to be some mechanism, based on chemical logic, to help recruit the RNA polymerase to the start of the protein-coding gene. Likewise, if this process is to be regulated, there needs to be some mechanism, or mechanisms to dictate when an RNA polymerase should be recruited to the start of a gene, when it should not, and/or if it is recruited to the DNA, whether it should begin transcription and how many times this process should happen. Note, that the previous sentence, has several distinct subproblems/questions (e.g. when is the polymerase recruited?, if recruited should it start transcription?, if it starts transcription, how many times should this process repeat?). We can also reasonably infer, that there will need to be some mechanisms to "instruct" (more anthropomorphisms) the polymerase to stop transcription. Recruiting RNA polymerase to specific sitesTo initiate transcription, the RNA polymerase must be recruited to a segment of DNA near the start of a region of DNA encoding a functional transcript. The function of the RNA polymerase as described so far, however, is not to bind specific sequences but to move along any segment of DNA. Recruiting the polymerase to a specific site therefore seems contradictory to its usual behavior. Explaining this contradiction requires us to invoke something new. Either transcription can start anywhere and just those events that lead to a full productive transcript do anything useful or something other than the RNA polymerase itself helps to recruit the enzyme to the beginning of a gene. The latter, we now take for granted, is the case. |
||||||||||||||||||||||||||||||
|
So let’s get back to the idea of switches. We can think of switches that are responsible for regulating gene expression. The expression of a gene or the transcription of a gene. And so, we can think of kind of basic on-off models first. Just like our simple light switches. And so, on the top here I've got a model, one where I've got some sort of double-stranded piece of DNA and here's a promoter. And in the model is a switch, a protein that comes and reversibly binds to the promoter. In this case this protein binding to the promoter blocks the promoter from the RNA polymerase and leads to no transcription. When the protein falls off the promoter transcription can turn on. This is one mode of regulation, which is kind of binary. The other way you might consider this working is you have some protein that comes binds the promoter and leads to enhanced transcription, the figure below. So in this model on top we have transcription without the protein. And then when the protein comes in and “Acts”, there is no transcription. And then model two, below, you have very little transcription from what might be a weak promoter- remember we talked about strong and weak promoters on Monday - and this promoter is turned “on” by the activity of a protein. These modes, mechanisms of transcriptional regulation, we'll give them names. One is called the negative mode of regulation; the other one is called a positive mode. Whether it's negative or positive refers to what happens when a particular protein binds at or near the promoter. So in this case, the binding of this protein has a negative effect on transcription. It turns it off or down. And the case on the bottom, the binding of this protein has a positive effect. It turns it up, at least when compared to what happens when that protein is not there. To repeat, we've got this protein-based binary mechanism that we can start use to regulate transcription at sites at or near promoters. How? By binding proteins to those sites. And the effect that those proteins can have can either be positive or negative on whatever the basal rate of that promoter is. In this case, you might have a strong promoter. It's doing its thing just fine. And we need to turn it down every now and then. Here we have a weak promoter that needs to be turned up every now and then. Any questions about the basic setup? Yes. When I say increases the effectiveness, does that mean it increases the ability to bind to the promoter? So, with this kind of scheme one thing that can play is fairly easy to imaging? You have a promoter that the RNA polymerase has no obstruction to binding to. It's a strong promoter. On its own it does a really good job of recruiting the RNA polymerase and starting transcription. Then when this other protein comes and binds, it actually just acts as a physical barrier so that the RNA polymerase can't access the promoter. So that's one kind of mechanism. And then the other mechanism I think you were proposing was here. The RNA polymerase has access, but the promoter itself doesn't really foster really good binding of the RNA polymerase. And so, most of the time comes to bind, it falls off. Having a protein nearby can, through various interactions with the polymerase help stabilize the polymerase on the promoter and to initiate transcription. Yes? The proteins are called transcription factors. We're gonna get to that. I just wanted to, right now , introduce the basic mechanism. So yes, they're called transcription factors. And they can either act positively or negatively. Alright. |
A second way to classify promoters by the use of the term activated or equivalently induced. These interchangeable terms are used to describe promoters sensitive to some external stimulus and respond to said stimulus by increasing transcription. Activated promoters have a base state exhibits little to no transcription. Transcription is then "activated" in response to a stimulus - the stimulus turns the promoter "on". Transcription factors help to regulate the behavior of a promoter How are promoters sensitive to external stimuli? Mechanistically, in both activation and repression require regulatory proteins to change the transcriptional output of the gene being observed. The proteins responsible for helping to regulate expression are generally called transcription factors. The specific DNA sequences bound by transcription factors are often called operators and most times the operators are very close to the promoter sequences. Why are the classifications of activator and repressor potentially problematic? These terms describe the proteins with idealized single functions. While this may be true with some transcription factors, in reality other transcription factors may act to activate gene expression in some conditions while repressing in other conditions. Some transcription factors will act to modulate expression either up or down depending on context rather than shutting transcription "off" or turning it completely "on". To circumvent some of this confusion, some of your instructors prefer to avoid using the terms activator and repressor and instead prefer to discuss the activity of transcription various transcription factors as either a positive or a negative influence on gene expression in specific cases. If we use these terms, you might hear your instructor saying that the transcription factor in question ACTS LIKE/AS a repressor or that it ACTS LIKE/AS an activator, taking care not to call it simply an activator or repressor. It is more likely, however, that you will hear them say that a transcription factor is acting to positively or negatively influence transcription. |
||||||||||||||||||||||||||||||
|
So what kind of physical factors? We just talked about that, right? You question, led us right into this slide. This idea here is that in the negative mode, the transcription factor is effectively just acting to block the RNA polymerase. |
Not covered in textbook | ||||||||||||||||||||||||||||||
|
So, let's take a look at this setup here. I need to be able to point from a different direction. Alright, so we've got a set of genes represented by the green boxes here. And these genes encode enzymes that can metabolize some food source, let's say glucose, or doesn't really matter what that food sources is; the organism is hungry! And to eat a nice meal, it needs to express these genes as proteins, enzymes in a metabolic pathway so that it can process the food it eats. We've got a potential source of food here. I'm going to speculate that the potential source of food is sporadically present, meaning that this organism encounters the food every so often, but not all that often. So how might we use these ideas, and the ones we just talked about, to regulate these genes? Yeah. So, you're proposing positive regulation in which a transcription factor would come and bind when needed, right? And turn on these genes just when they're needed. That could work. Any other ideas? You’re not wrong, by the way. There just may be multiple solutions. Can we use negative regulation somehow? Yeah? There was a proposal use positive regulation and I'm wondering, can we also use negative regulation and how might that work? |
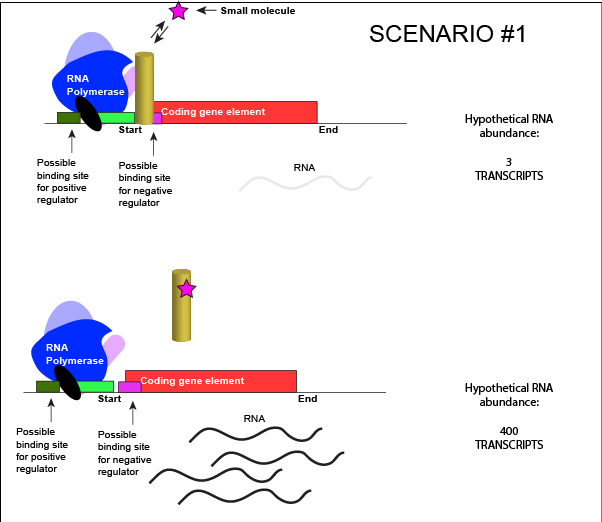
Allosteric Modulators of Regulatory ProteinsThe activity of many proteins, including regulatory proteins and various transcription factors, can be allosterically modulated various factors, including by the relative abundance of small molecules in the cell. We often refer these small molecules as inducers or co-repressorsor co-activators and are often metabolites, such as lactose or tryptophan or small regulatory molecules, such as cAMP or GTP. These interactions allow the TF to respond to environmental conditions and to modulate its function accordingly. It helps to decide whether to transcribe a gene depending on the abundance of the environmental signal. Let us imagine a negative transcriptional regulator. In the most simple case we've considered so far, transcription of a gene with a binding site for this transcription factor would be low when the TF is present and high when the TF is absent. We can now add a small molecule to this model. Here the small molecule can bind the negative transcriptional regulator through sets of complementary hydrogen and ionic bonds. In this first example we will consider the case where the binding of the small molecule to the TF induces a conformational change to the TF that severely reduces its ability to bind DNA. If this is the case, the negative regulator - once bound by its small molecule - would release from the DNA. This would thereby relieve the negative influence and lead to increased transcription. This regulatory logic might be appropriate to have evolved in the following scenario: a small molecule food-stuff is typically absent from the environment. Therefore, genes encoding enzymes that will degrade/use that food should be kept "off" most of the time to preserve the cellular energy that their synthesis would use. A negative regulator could accomplish this. When the food-stuff appears in the environment, it would be appropriate for the enzymes responsible for its processing to be expressed. The food-stuff could then act by binding to the negative regulator, changing the TF's conformation, causing its release from the DNA and turning on transcription of the processing enzymes.
An abstract model of a generic transcriptional unit regulated by a negative regulator whose activity is modulated by a small molecule (depicted by a star). Here, the binding of the small molecule causes the TF to release from the DNA. Attribution: Marc T. Facciotti (own work) We can consider a second model for how a negatively acting TF might interact with a small molecule. Here, the TF alone cannot bind its regulatory site to the DNA. However, when a small molecule binds to the TF a conformational change occurs that reorients DNA binding amino-acids into the "correct" orientation for DNA binding. The TF-small molecule complex now binds to the DNA and acts to influence transcription negatively.
An abstract model of a generic transcriptional unit regulated by a negative regulator whose activity is modulated by a small molecule (depicted by a star). Here, binding of the small molecule causes the TF to bind to the DNA. Attribution: Marc T. Facciotti (own work) Note how the activity of the TF can be modulated in distinctly different ways by a small molecule. Depending on the protein, the binding of this external signal can either cause binding of the TF-small molecule complex to DNA OR binding of the small molecule can cause the release of the TF-small molecule complex from the DNA. We can work up the same kinds of examples for a positive regulator. |
||||||||||||||||||||||||||||||
|
So let, let me draw your model on the board and we'll also draw the positive one on the board too. We’ll start with the negative one real quick. So I have, I'm going to just put one gene here. We'll put a transcriptional terminator down there. Let's make this the initiation site and the promoter. Alright, and you're proposing, go through it again real quick. I have a protein and then the food source, a little small molecule. And they can have some equilibrium where that protein is bound and it exists in an unbound form as well. So when there's a lot of the food source that goes to the bound form. Okay? Good. And then what you're saying, I think what I heard you say was that when the protein is in it's unbound form, it is able to go and bind the promoter and negatively regulate the gene. And then when there's a lot of this food source, it binds to the protein and it reverts to this small-molecule-bound form which does not bind the promoter. Yeah. Okay, I like that. |
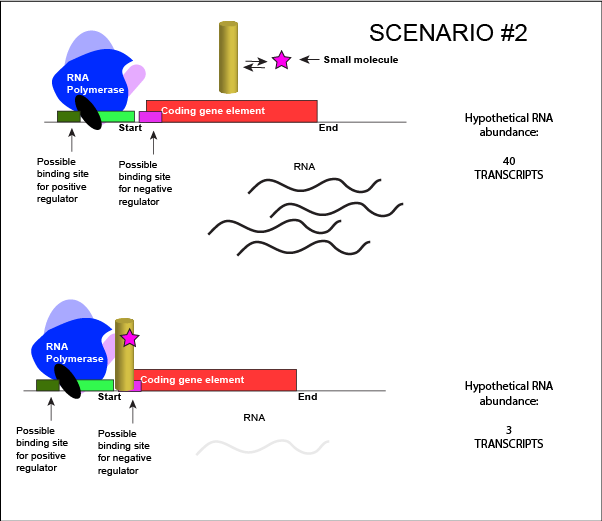
Allosteric Modulators of Regulatory ProteinsThe activity of many proteins, including regulatory proteins and various transcription factors, can be allosterically modulated various factors, including by the relative abundance of small molecules in the cell. We often refer these small molecules as inducers or co-repressorsor co-activators and are often metabolites, such as lactose or tryptophan or small regulatory molecules, such as cAMP or GTP. These interactions allow the TF to respond to environmental conditions and to modulate its function accordingly. It helps to decide whether to transcribe a gene depending on the abundance of the environmental signal. Let us imagine a negative transcriptional regulator. In the most simple case we've considered so far, transcription of a gene with a binding site for this transcription factor would be low when the TF is present and high when the TF is absent. We can now add a small molecule to this model. Here the small molecule can bind the negative transcriptional regulator through sets of complementary hydrogen and ionic bonds. In this first example we will consider the case where the binding of the small molecule to the TF induces a conformational change to the TF that severely reduces its ability to bind DNA. If this is the case, the negative regulator - once bound by its small molecule - would release from the DNA. This would thereby relieve the negative influence and lead to increased transcription. This regulatory logic might be appropriate to have evolved in the following scenario: a small molecule food-stuff is typically absent from the environment. Therefore, genes encoding enzymes that will degrade/use that food should be kept "off" most of the time to preserve the cellular energy that their synthesis would use. A negative regulator could accomplish this. When the food-stuff appears in the environment, it would be appropriate for the enzymes responsible for its processing to be expressed. The food-stuff could then act by binding to the negative regulator, changing the TF's conformation, causing its release from the DNA and turning on transcription of the processing enzymes.
An abstract model of a generic transcriptional unit regulated by a negative regulator whose activity is modulated by a small molecule (depicted by a star). Here, the binding of the small molecule causes the TF to release from the DNA. Attribution: Marc T. Facciotti (own work) We can consider a second model for how a negatively acting TF might interact with a small molecule. Here, the TF alone cannot bind its regulatory site to the DNA. However, when a small molecule binds to the TF a conformational change occurs that reorients DNA binding amino-acids into the "correct" orientation for DNA binding. The TF-small molecule complex now binds to the DNA and acts to influence transcription negatively.
An abstract model of a generic transcriptional unit regulated by a negative regulator whose activity is modulated by a small molecule (depicted by a star). Here, binding of the small molecule causes the TF to bind to the DNA. Attribution: Marc T. Facciotti (own work) Note how the activity of the TF can be modulated in distinctly different ways by a small molecule. Depending on the protein, the binding of this external signal can either cause binding of the TF-small molecule complex to DNA OR binding of the small molecule can cause the release of the TF-small molecule complex from the DNA. We can work up the same kinds of examples for a positive regulator. |
||||||||||||||||||||||||||||||
|
And then let's go through the positive scenario. Same gene, different mechanism. |
Let us imagine a negative transcriptional regulator. In the most simple case we've considered so far, transcription of a gene with a binding site for this transcription factor would be low when the TF is present and high when the TF is absent. We can now add a small molecule to this model. Here the small molecule can bind the negative transcriptional regulator through sets of complementary hydrogen and ionic bonds. In this first example we will consider the case where the binding of the small molecule to the TF induces a conformational change to the TF that severely reduces its ability to bind DNA. If this is the case, the negative regulator - once bound by its small molecule - would release from the DNA. This would thereby relieve the negative influence and lead to increased transcription. This regulatory logic might be appropriate to have evolved in the following scenario: a small molecule food-stuff is typically absent from the environment. Therefore, genes encoding enzymes that will degrade/use that food should be kept "off" most of the time to preserve the cellular energy that their synthesis would use. A negative regulator could accomplish this. When the food-stuff appears in the environment, it would be appropriate for the enzymes responsible for its processing to be expressed. The food-stuff could then act by binding to the negative regulator, changing the TF's conformation, causing its release from the DNA and turning on transcription of the processing enzymes.
An abstract model of a generic transcriptional unit regulated by a negative regulator whose activity is modulated by a small molecule (depicted by a star). Here, the binding of the small molecule causes the TF to release from the DNA. Attribution: Marc T. Facciotti (own work) We can consider a second model for how a negatively acting TF might interact with a small molecule. Here, the TF alone cannot bind its regulatory site to the DNA. However, when a small molecule binds to the TF a conformational change occurs that reorients DNA binding amino-acids into the "correct" orientation for DNA binding. The TF-small molecule complex now binds to the DNA and acts to influence transcription negatively.
An abstract model of a generic transcriptional unit regulated by a negative regulator whose activity is modulated by a small molecule (depicted by a star). Here, binding of the small molecule causes the TF to bind to the DNA. Attribution: Marc T. Facciotti (own work) Note how the activity of the TF can be modulated in distinctly different ways by a small molecule. Depending on the protein, the binding of this external signal can either cause binding of the TF-small molecule complex to DNA OR binding of the small molecule can cause the release of the TF-small molecule complex from the DNA. We can work up the same kinds of examples for a positive regulator. |
||||||||||||||||||||||||||||||
|
Where was the positive idea? How does the positive work in your case? You're proposing a weak promoter here. And then there's a food source that can bind to the protein Then what happens? This one then binds to the promoter, Yeah, the small-molecule-bound form of the protein. Alright, so each of these mechanisms is fine, right? You can have both. You can use positive regulation to turn on these genes. And we kind of skipped ahead a little bit because of your nice comments, but the ideas are right. Also some of these questions of when and how fit together, right? So when? In this case, we need to turn these genes on, when the food is around. If the food is not around. It's a waste of energy to turn on the genes. Somebody said, to make the proteins, to make these transcripts. We don't need to make them, when the food isn’t around. One way, the how, of making a decision of whether to do something when the food is around or not is to use allosteric binding with a protein. I see the hand in the front. Here, where the small molecule is binding the protein we have an example of allosteric regulation. The food is probably not binding the protein where it's binding the DNA and the food binding influences its ability of the transcription factor to bind the DNA. This is just like when we talked about metabolic regulation, the small molecule being able to bind and enzyme somewhere not in the active site and influencing the ability of that enzyme to do its job. There was a question. So, the question is, now that you've got both schemes and both are possible, which one do you choose? Is it more energetically favorable to do the negative? Or is it more energetically favorable to use the positive mode? We're going to answer that in a moment. It turns out the answer may not have to do with strictly with energy, but it's still debated. For now let’s just agree that you can theoretically use both modes to regulate. And nature actually employs both modes. It turns out that nature seems to have a preference depending on what the situation is. We'll get to that in a second. |
Let us imagine a negative transcriptional regulator. In the most simple case we've considered so far, transcription of a gene with a binding site for this transcription factor would be low when the TF is present and high when the TF is absent. We can now add a small molecule to this model. Here the small molecule can bind the negative transcriptional regulator through sets of complementary hydrogen and ionic bonds. In this first example we will consider the case where the binding of the small molecule to the TF induces a conformational change to the TF that severely reduces its ability to bind DNA. If this is the case, the negative regulator - once bound by its small molecule - would release from the DNA. This would thereby relieve the negative influence and lead to increased transcription. This regulatory logic might be appropriate to have evolved in the following scenario: a small molecule food-stuff is typically absent from the environment. Therefore, genes encoding enzymes that will degrade/use that food should be kept "off" most of the time to preserve the cellular energy that their synthesis would use. A negative regulator could accomplish this. When the food-stuff appears in the environment, it would be appropriate for the enzymes responsible for its processing to be expressed. The food-stuff could then act by binding to the negative regulator, changing the TF's conformation, causing its release from the DNA and turning on transcription of the processing enzymes.
An abstract model of a generic transcriptional unit regulated by a negative regulator whose activity is modulated by a small molecule (depicted by a star). Here, the binding of the small molecule causes the TF to release from the DNA. Attribution: Marc T. Facciotti (own work) We can consider a second model for how a negatively acting TF might interact with a small molecule. Here, the TF alone cannot bind its regulatory site to the DNA. However, when a small molecule binds to the TF a conformational change occurs that reorients DNA binding amino-acids into the "correct" orientation for DNA binding. The TF-small molecule complex now binds to the DNA and acts to influence transcription negatively.
An abstract model of a generic transcriptional unit regulated by a negative regulator whose activity is modulated by a small molecule (depicted by a star). Here, binding of the small molecule causes the TF to bind to the DNA. Attribution: Marc T. Facciotti (own work) Note how the activity of the TF can be modulated in distinctly different ways by a small molecule. Depending on the protein, the binding of this external signal can either cause binding of the TF-small molecule complex to DNA OR binding of the small molecule can cause the release of the TF-small molecule complex from the DNA. We can work up the same kinds of examples for a positive regulator. |
||||||||||||||||||||||||||||||
|
Alright, so I just drew this out on the board, but here it is for your, your notes, right? We can have a system in which RNA polymerase can be blocked. This is the negative system. And then when the small molecule, the food comes around, it binds allosterically to the regulator, the transcription factor, and then releases that transcription factor from the promoter or regions around the promoter and allows the RNA polymerase to transcribe. The other mechanism is to talk about this positive regulation which we just drew up here, where in the absence of small molecule, the RNA polymerase doesn't bind the promoter, but when the food is around, the RNA polymerase will bind the promoter with the of a transcription factor that binds near the promoter, helps recruit the RNA polymerase and initiates transcription. Alright, so we can do both of these things. These are like smart light switches, right? Or like in some ways they’re like a thermostat. These transcription factors, through their allosteric binding sites they are reading the concentration of small molecule. The more of a small molecule there is, if it's a negative regulator, the more it's going to release the transcription factor from the promoter, the less small moleule there is, the more the transcription factor is going to tend to bind the promoter. If it's a positive regulator this works in reverse. Alright, so we can use both of these modes to regulate transcription. And they're smart sensors. Now, the other thing I want you guys to note is that we're again reloading various lessons. If I'm putting my instructors hat on, what I am seeing here are fantastic exam questions that asked you not only about whether or not you understand the basic mechanisms of how a protein might be used to turn on or off a gene, but also whether or not you're making a link to the topics we've seen before. So, the small molecule binding to an allosteric site, we've seen that somewhere else before. That's a theme that we’re now revisiting in a different context. If you guys remember from the very beginning of class, we had our protein plus small molecule in some sort of binding equilibrium with the small molecule. And we had all sorts of little graphs and charts to describe that. And what I was looking for was, among other things, was for you guys to start being able to zoom into pictures like this and to understand that there were interactions between functional groups taking place and that those interactions can be modulated by environmental factors. So, this is very much the same story as eight weeks ago. We’re just revisiting it in the context of turning genes on and off. Okay, good. I’m not sure what might be done with text like this. This is something that is clearly only from lecture, I think. It is the instructor stepping back and explicitly telling the students what concepts from the course they are hoping that the students will connect together. It seems like something that should be able to point to previous slides or exercises or readings - I know for example that this stuff is connected in the learning objective network for instance. Anyhow, it’s important enough that the instructor is making a special note of it. |
Is it positive or negative regulation?Resolving a common point of confusion It is not uncommon for many Bis2a students to be slightly confused about how to determine if a transcription factor is acting as a positive or negative regulator. This confusion often comes after a discussion of the modes that the stimulus (i.e. small molecule) can influence the activity of a transcription factor. This is not too surprising. In the examples above, the binding of an effector molecule to a transcription factor could have one of two different effects: (1) binding of the effector molecule could induce a DNA-bound transcription factor to release from its binding site, derepressing a promoter, and "turning on" gene expression. (2) binding of the effector molecule to the transcription factor could induce the TF to bind to its DNA binding site, repressing transcription, and "turning off" gene expression. In the first case, it might appear that the TF is acting to regulate expression positively, while in the second example it might appear that the TF acts negatively. A genetic test for positive or negative regulatory function of a TFHow does one determine if a regulatory protein functions in a positive or negative way? A simple genetic test is to ask "what happens to expression if the regulatory protein is absent?" This can be accomplished by removing the coding gene for the transcription factor from the genome. If a transcription factor acts positively, then its presence is required to activate transcription. In its absence, there is no regulatory protein, therefore no activation, and the outcome is lower transcription levels of a target gene. The opposite is true for a transcription factor acting negatively. In its absence expression should be increased, because the gene keeping expression low is no longer around. |
||||||||||||||||||||||||||||||
|
Let’s look at a specific example now that we've got the basic ideas of the mechanistic options we can use for regulating the expression of particular genes. We'll look at a specific example of food. And that food is lactose. And we're going to pick E. Coli is the model system to better understand how these kind of basic mechanisms for transcriptional regulation can work. We pick E. coli system because it is maybe the best understood system in biology, certainly when it comes to how transcription regulation works. And here actually multiple transcription factors work to regulate the expression of a genes in response to some environmental signal, in this case, the presence or absence of food. Yeah, question? Is it possible that the positive and negative transcriptional mechanisms are both used to control the expression of the same gene. Yes. We're about to see that with lactose. Another reason to pick this example is that it gives us the first view, with a kind of an easy example, of how a system can integrate multiple sources of information to make a decision. So for example, you might want to know whether lactose is available, but if there's something else that you prefer eating over the lactose, maybe you'd like to know if that's around and that helps you make a decision about whether to transcribe certain genes are not. In this case you need to sense multiple things and integrate that information at the promoter to make a decision about whether to transcribe certain genes. Now, remember, this is a simple system. All we're doing right now is laying down basic ideas. What is the basic mechanism? What is the basic way that these mechanisms work? How can we combine them to do fancy things like sense multiple things in the environment. And then when you take more advanced courses, you will start looking at systems that integrate not one piece of information, not two pieces of information, but tens of different sources of information through all sorts of crazy pathways. And all that information gets filtered down to a gene where then you use positive and negative modes of regulation together to figure out whether to express it or not. So very cool stuff. But let's look first at the simple example for lactose. So, lactose is a disaccharide. If I put this molecule on the final exam, you'd be able to put this in the carbohydrate and lipid bin, not in the nucleic acid, lipid or protein bin. It's a disaccharide of galactose and glucose. We're quite familiar with it from its presence in milk and other dairy kind of stuff. |
Example #2: The lac operonRationale for studying the lac operonIn this example, we examine the regulation of genes encoding proteins whose physiological role is to import and assimilate the disaccharide lactose, the lac operon. The story of regulating lac operon is a common example used in many introductory biology classes to illustrate basic principles of inducible gene regulation. We describe this example second because it is, in our estimation, more complicated than the previous example involving the activity of a single negatively acting transcription factor. By contrast, the regulation of the lac operon is a wonderful example of how the coordinated activity of both positive and negative regulators around the same promoter can integrate multiple different sources of cellular information to regulate the expression of genes. The utilization of lactoseLactose is a disaccharide composed of the hexoses glucose and galactose. We commonly encounter lactose in milk and some milk products. As one can imagine, the disaccharide can be an important food-stuff for microbes that can use its two hexoses. E. coli can use multiple different sugars as energy and carbon sources, including lactose and the lac operon is a structure that encodes the genes necessary to acquire and process lactose from the local environment. E. coli, however, does not frequently encounter lactose, and therefore the genes of the lac operon must typically be repressed (i.e. "turned off") when lactose is absent. Driving transcription of these genes when lactose is absent would waste precious cellular energy. By contrast, when lactose is present, it would make logical sense for the genes responsible for using the sugar to be expressed (i.e. "turned on"). So far, the story is very similar to that of the tryptophan operon described above. |
||||||||||||||||||||||||||||||
|
A number of years ago, back in the '50s, there were some pioneering experiments done with E coli, where scientists were studying what E coli like to eat. So they would feed E. coli different sugars and they'd say, “Well, how fast does it grow on this sugar, and how fast is it growing on another sugar? Which does it seem to prefer? They concluded, “Well, it seems to grow better or worse on different sugars.” Then they asked, “What happens if we mix the sugars together and make the organism make a decision?” Or ask whether it makes a decision like: “Do I like one sugar better than the other?” These experiments were done by putting E coli into a little tube and then adding two sugars that you want to try and consider as something for the E. coli to dine on. And then they monitor here on the y-axis the log of the bacterial culture mass, or basically the growth. How much of how much of the bacterial culture is there? And you monitor that over time. So, they were looking at cell division as a reflection of how well the cell is doing while eating a particular mix of sugars. And what did they noticed E coli doing? And this is just one of the experiments that they did. But you see this behavior for a lot of different combinations of sugars. Here they gave E. coli glucose and lactose. What they found was E coli would grow really nicely. And then the culture would reach this point where it stopped growing for a little while. And then started growing again before reaching some plateau where it didn’t grow anymore. They also started measuring the concentrations of the various sugars that were in the media during growth. Like what is it eating and when? What's disappearing? And they found that almost entirely, at the beginning, the organism was eating glucose. And then there was a period here in which it had to make a decision to say, Okay, now I'm done eating glucose, but I sense that there's something else around. And I need to turn the genes on that are responsible for eating that other thing. There's a little lag in growth observed while the organism adapts to the new food that is going to eat. And then it starts eating the lactose until it runs out of all the sugars and can't grow anymore. Comments? Question? Okay. Alright, Everybody ok with that? These were experiments done by people that are no longer with us, Jacob and Monod in France back in the 1950s. Alright, and they're classic because they are one of the first times where you see an organism like making a discrete choice and changing expression of genes in order to respond to the changing environment. |
However, there is a catch. Experiments conducted in the 1950's by Jacob and Monod showed that E. coli prefers to use all the glucose present in the environment before it uses lactose. This means that the mechanism used to decide whether or not to express the lactose utilization genes must be able to integrate two types of information (1) the concentration of glucose and (2) the concentration of lactose. While this could theoretically be accomplished in multiple ways, we will examine how the lac operon accomplishes this by using multiple transcription factors. | ||||||||||||||||||||||||||||||
|
Alright, so one more piece of information I need to give you guys so that we can have the rest of the discussion on this regulatory mechanism is that there's a compound that's important in almost all cells, at least all that I know of. That molecule is called cyclic AMP or abbreviated cAMP. It is used by many cells as an indicator of energy status. Like, when cyclic AMP is abundant, it indicates a kind of low energy status for the cell. And when it's at a low concentration, it indicates a high energy status. cAMP is made by taking ATP and an enzyme called adenylate cyclase. The enzyme dephosphorylates, liberates a pyrophosphate, here, and uses that energy to make a covalent link between the remaining phosphate and its own free three-prime hydroxyl. That results in this cyclic bond here, and so we give this the name cyclic AMP. And without going into all the detail, what we find in E. Coli is when there's a lot of glucose around - if you go in and measure the concentration of this molecule, cyclic AMP, there's low cyclic AMP. When there's low glucose transport and low glucose around, we have high amounts of cyclic AMP. If we want to – and this is not a 100% correct way to do it, but it's a convenient little trick for your brain - if we think about glucose and glycolysis; if glycolysis is running, it gives the cell some energy. If the cell has got high glucose, we think it makes a lot of ATP. That means we will have low AMP. And if we don't have a lot of glucose, we might be energy starved. And so, we won't have a lot of ATP, but rather a lot of it might be in this other form, this low-energy AMP form. Again, this story is not 100% mechanistically connect, but I find it a convenient way of remembering what the relationship between glucose and cAMP concentrations are. Question? No, I won't ask you on that on the exam. |
CAP protein - an indirect sensor of glucoseIn E. coli, when glucose levels drop, the small molecule cyclic AMP (cAMP) accumulates in the cell. cAMP is a common signaling molecule that is involved in glucose and energy metabolism in many organisms. When glucose levels decline in the cell, the increasing concentrations of cAMP allow this compound to bind to the positive transcriptional regulator called catabolite activator protein (CAP) - also referred to as CRP. cAMP-CAP complex has many sites throughout the E. coli genome and many of these sites are located near the promoters of many operons that control the processing of various sugars. |
||||||||||||||||||||||||||||||
|
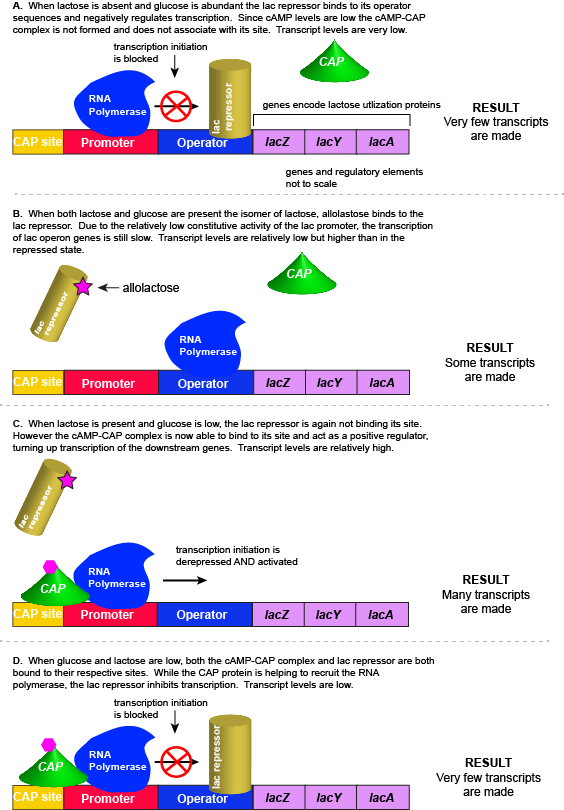
Alright, so here's the setup for the lactose utilization genes. We have a set of genes labeled lac, Z, Y and A that are preceded by this site called 0. And here I'll introduce the O. The O is called an operator. An operator is the binding site for a transcription factor. So, we've got the promoter, which is strictly the binding site for the RNA polymerase. And then we've got other signal sequences around that promoter that we call operators. They recruit transcription factors, either positively or negatively acting. Sometimes these operators overlap the promoters a little bit. Other times they're quite far away and still act to regulate the expression of a gene. Alright, and then there's another gene here that's got its own promoter, placI, which encodes this gene. It gets transcribed and translated into this transcription factor here called lacI. LacI is a negative regulator. It binds its operator here near, it's actually got three operators, but it binds an operator near the lac promoter. And in so doing as a negative regulator, right, stops the RNA polymerase from binding its promoter and transcribing the genes for lactose utilization. By the way, lac Y is a transporter for lactose. Alright. So, just like we drew on the board, lacI is a negative regulator that has allosteric binding capability for lactose. When lactose is around, actually in the form of allolactose, in high concentration it can bind this negative regulator, causing it to release from its operator and allowing the RNA polymerase to go through and transcribe and make an mRNA out of these three genes. And those can be translated into the enzymes that are then responsible for using the lactose in the environment. Alright, so this is like the model that we looked at here, right? Where the transcription factor binds until a small molecule comes around. And when the small molecule comes around and shifts it to a form that can't bind the promoter anymore transcription can then happen. This is a negative regulatory mechanism. Alright, everybody, okay with that one so far? By the way, let me make a plea to you guys. I have a colleague who refuses to teach the regulation of the lac operon. And, I understand exactly why she won't do it. Which is because everybody is then tempted to memorize the details of this specific example. This happens for multiple reasons. One is, this is what we do. There's a strong tradition in high school biology courses of basically teaching this material and then asking students very specific questions about the mechanism of lactose regulation in E. Coli. And none of us in intro biology really care about those specifics very much. What we care about is that we use this as an example to learn more generally about how regulation can work mechanistically. So we're taking a specific example, whose details I will not test you on. I’m not going to have the lac repressor on an exam. I'm not going to have lac Z, Y, and A. There are hundreds of sites in the E coli genome that work using the same mechanistic principles. So, we're using this one best understood example to illustrate and study mechanism and not the specific example. Question? Yeah. Is there a separate RNA polymerase for placI and pLac? Yes. This promoter here, recruiting RNA polymerase transcribes and translate transcribes pLacI or the lac repressor. That happens independently of the transcription and the regulation here. They are nearby to one another in the genome though, what this is depicting is the protein to regulate the genes that eat lactose is also encoded the gene to regulate the proteins that regulate the expression of the synthesis of those genes. This promoter is not under the same control as the pLac promoter. You can think of it as constitutive promoter. It's always making a basal level of this transcription factors. There’s always some LacI around. And then the cell can decide once LacI is round of whether or not to regulate pLac or not. Excellent. |
States of a regulated promoterSince promoters recruit an RNA polymerase these sites and the assembly of the pre-initiation complex are obvious sites for regulating the first steps of gene expression. At the level of transcription initiation, we often classify promoter into one of three classes. We call the first constitutive. Constitutive promoters are generally not regulated very strongly. Their base state is "on". When the constitutive transcription from a promoter is very high (relative to most other promoters), we will colloquially call that promoter a "strong constitutive" promoter. If the amount of transcription from a constitutive promoter is low (relative to most other promoters) we call that promoter a "weak constitutive" promoter. The transcriptional regulators of the lac operonThe lac repressor - a direct sensor of lactoseAs noted, the lac operon normally has very low to no transcriptional output in the absence of lactose. This is because of two factors: (1) the constitutive promoter strength for the operon is relatively low and (2) the constant presence of the LacI repressor protein negatively influences transcription. This protein binds to the operator site near the promoter and blocks RNA polymerase from transcribing the lac operon genes. By contrast, if lactose is present, lactose will bind to the LacI protein, inducing a conformational change that prevents LacI-lactose complex from binding to its binding sites. Therefore, when lactose is present, the negative regulatory LacI is not bound to its binding site and transcription of lactose using genes can proceed. |
||||||||||||||||||||||||||||||
|
Alright, so the second part of this story is that it doesn't end there, right? So we've got this idea of negative regulation. There's another, it turns out, factor that's involved in regulating the expression of these genes and that is this transcription factor called CRP. CRP is sensitive to cyclic AMP concentration. This transcription factor exists in two forms, a cAMP-unbound form and a cAMP-bound form here. So unbounded it’s got its legs crossed here. It's got just standing straight. And in the cAMP-bound form, it can uncross its legs and go bind an operator sequence near the pLac promoter, helping the RNA polymerase, in this case, transcribe the genes responsible for lactose utilization. So, the genes that are responsible for utilizing lactose are sensitive to both lactose and indirectly glucose through the molecule cyclic AMP. |
CAP protein - an indirect sensor of glucoseIn E. coli, when glucose levels drop, the small molecule cyclic AMP (cAMP) accumulates in the cell. cAMP is a common signaling molecule that is involved in glucose and energy metabolism in many organisms. When glucose levels decline in the cell, the increasing concentrations of cAMP allow this compound to bind to the positive transcriptional regulator called catabolite activator protein (CAP) - also referred to as CRP. cAMP-CAP complex has many sites throughout the E. coli genome and many of these sites are located near the promoters of many operons that control the processing of various sugars. |
||||||||||||||||||||||||||||||
|
Alright, so let's, let's put some of this logic together. The organism is making a choice. In this case, it's got glucose and lactose around at the beginning. But it's not turning on the genes for lactose utilization until glucose is gone. So, how's it doing this? It's asking itself two things. It's asking itself, is lactose around and is glucose around? It's sensing those two pieces of information with allosteric binding of molecules to transcription factors. It’s measuring if lactose is around with the lac repressor. And it says if lactose is around, “Hey, I might want to use lactose, I'm going to unblock the operator and maybe allow transcription to happen.” It turns out that this is a very weak promoter. So, it's unlocking a weak promoter which will transcribe those genes, but not very strongly. And that's good because if there's also a lot of glucose around, it will eat the glucose and not turn these genes on, even though the lactose is there. Once the glucose runs out, cyclic AMP levels go up. The cyclic AMP and CRP together act as a positive regulator to then make up for that weak promoter and drive the transcription so that the organism can utilize lactose. When we come back, we'll play more games with these positive and negative regulators. Remember, focus on the mechanism. We use the lac operon as an example, not as the law of all things that you need to memorize. |
Putting it all together: Inducing expression of the lac operonFor the lac operon to be activated, two conditions must be met. First, the level of glucose must be very low or non-existent. Second, lactose must be present. Only when glucose is absent and lactose is present, will the lac operon be transcribed. When this condition is achieved the LacI-lactose complex dissociates the negative regulator from near the promoter, freeing the RNA polymerase to transcribe the operon's genes. High cAMP (indirectly indicative of low glucose) levels trigger the formation of the CAP-cAMP complex. This TF-inducer pair now bind near the promoter and act to positively recruit the RNA polymerase. This added positive influence boosts transcriptional output and lactose can be efficiently utilized. The mechanistic output of other combinations of binary glucose and lactose conditions are described in the table below and in the figure that follows.
Truth Table for Lac Operon
Transcription of the lac operon is carefully regulated so that its expression only occurs when glucose is limited and lactose is present to serve as an alternative fuel source. |
||||||||||||||||||||||||||||||
| Not covered in lecture |
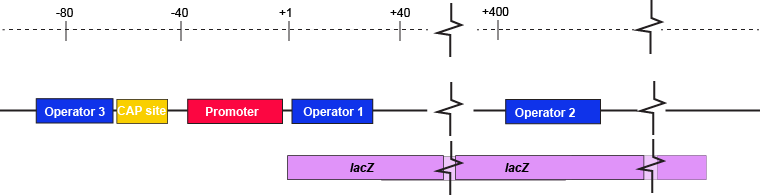
A more nuanced view of lac repressor functionThe description of the lac repressor's function correctly describes the logic of the control mechanism used around the lac promoter. However, the molecular description of binding sites is a bit overly simplified. In reality, the lac repressor has three similar, but not identical, binding sites called Operator 1, Operator 2, and Operator 3. Operator 1 is very close to the transcript start site (denoted +1). Operator 2 is located about +400nt into the coding region of the LacZ protein. Operator 3 is located about -80nt before the transcript start site (just "outside" of the CAP binding site).
The lac operon regulatory region depicting the promoter, three lac operators, and CAP binding site. The coding region for the Lac Z protein is also shown relative to the operator sequences. Note that two of the operators are in the protein coding region - there are multiple different types of information simultaneously encoded in the DNA. Attribution: Marc T. Facciotti (own work)
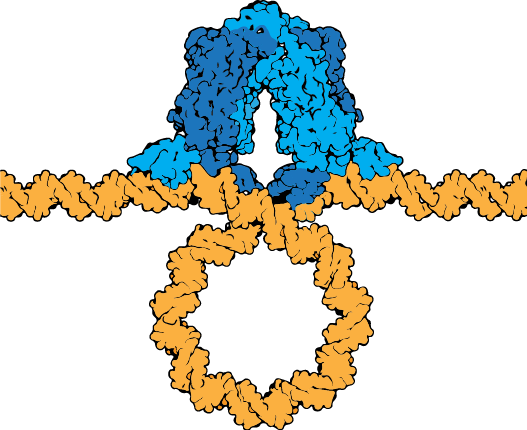
The lac repressor tetramer (blue) depicted binding two operators on a strand of looped DNA (orange). |
||||||||||||||||||||||||||||||












 Point
Point