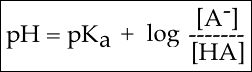2021_pH_pKA_for_Review
- Page ID
- 50639
The Role of Acid/Base Chemistry in General Biology
We have learned that the behavior of chemical functional groups depends on the composition, order, and properties of their constituent atoms. We will see that pH, a measure of the hydrogen ion concentration of a solution, can alter the chemical properties of some key biological functional groups in ways that change how they interact with other molecules and thus their biological role.
For example, depending on the pH, some functional groups on the amino acid that make up proteins can exist in different chemical states. We will learn that the chemical state of these functional groups can have a profound effect on the shape of the protein or on its ability to carry out chemical reactions. As we move through the course, we will see many examples of this type of chemistry in different contexts.
In pure water, hydrogen ions are spontaneously generated by the dissociation (ionization) of a small percentage of water molecules into equal numbers of hydrogen (H+) ions and hydroxide (OH-) ions. The OH- that result from the ionization of water departs into the sea of water molecules interacting with other molecules through polar interactions, while the now "free" (unbonded) H+ ions produced by the ionization associates with water molecules (line two of the figure below) to create a new molecule called a hydronium ion, H3O+. At some point, the hydroxide ion from line 1 in the figure below will rejoin with a proton and reform another water molecule. This process of dissociation and re-association between hydroxide and hydrogen ions happens continuously at equilibrium.
While most H+ ions in solution really exist as H3O+ ions, we usually represent the H3O+ in figures or equations more simply as H+. Why? Because it is easier. Just remember that since nearly all chemistry in biology happens in water that when you see H+ referred to in the text, figures, or in equations, it usually represents H3O+.
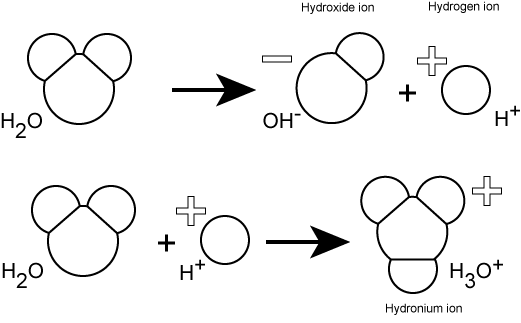
Figure 1: Water spontaneously dissociates into a proton and hydroxyl group. The proton will combine with a water molecule forming a hydronium ion.
Attribution: Marc T. Facciotti
While some paradoxes to this rule can be found in the chemistry of concentrated solutions, in General Biology, it is convenient to formally define pH as:
|
\[ pH = -\log_{10} [H^+]\]
|
In the equation above, the square brackets surrounding [H+] indicate concentration. If necessary, try a math review at wiki-logarithm or kahn-logarithm. Also see: definition-concentration or wiki-concentration. The pH of a solution is therefore a measure of the concentration of hydrogen ions in a solution (or the number of hydronium ions).
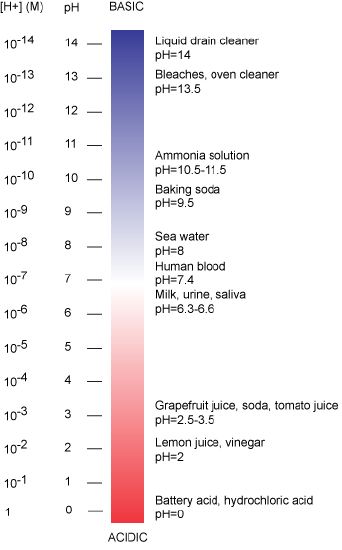
The pH is typically reported on a logarithmic pH scale that ranges from 0 to 14 (Figure 2). We define pH=7.0 as neutral. We call anything with a pH below 7.0 acidic and any reported pH above 7.0 alkaline or basic. Extremes in pH in either direction from 7.0 are often considered inhospitable to life, although examples exist to the contrary. pH levels in the human body usually range between 6.8 and 7.4, except in the stomach where the pH is more acidic, typically between 1 and 2. Some microbial species like Sulfolobus acidocaldarius thrive in hyper acidic environments (pH < 3) while others like Natronomonas pharaonis have been found living in lakes with pH > 11. These organisms are classified as "extremophiles" for their abilities to thrive in extreme environments. Proteins from these organisms are sometimes used in industrial processes where their ability to withstand environmental stress is a valued property.
Figure 2: The pH scale ranging from acidic to basic with various biological compounds or substances that exist at that particular pH. Attribution: Marc T. Facciotti
For Additional Information
Watch this video for an expanded explanation of pH and its relationship to [H+] and the logarithmic scale.
Let's work out an example to see how the pH scale works.
For reference: 1 mole (mol) of a substance (which can be atoms, molecules, ions, etc.), is defined as being equal to 6.02 x 1023 particles of the substance. Therefore, 1 mole of water is equal to 6.02 x 1023 water molecules.
Mathematically this can be written as:
1 mol = 6.02x1023 particles in a substance
1 mol H2O = 6.02x1023 water molecules
Example: The concentration of hydrogen ions dissociating from pure water is approximately 1 × 10-7 moles H+ ions per liter of water. The pH is calculated as the negative of the base 10 logarithm of this unit of concentration. The log10 of 1×10-7 is -7.0, and the negative of this number yields a pH of 7.0 (neutral pH).
Mathematically this can be represented as:
pH = -log10[H+]
pH = -log10[1×10-7]
pH = 7.0 (neutral pH)
The figure below provides another way to visualize the inverse relationship between proton and hydroxide ion concentrations by graphically illustrating how proton concentration decreases as pH increases while the hydroxide ion concentration simultaneously increases.
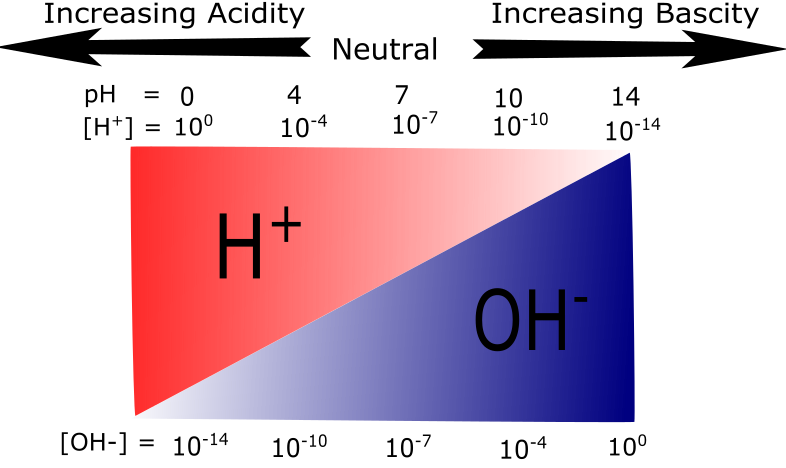
Figure 3: A graphical representation of acidity and basicity. This figure illustrates the relationship between H+ and OH- concentrations on the pH scale. At low pH values H+ ions are plentiful. As the pH increases the relative abundance of OH- ions increase while H+ abundance decreases.
Attribution: Mary O. Aina
The inverse relationship between pH and the concentration of protons confuses many students - take the time to convince yourself that you "get it." One way could be to predict whether different pH values are acidic or basic and then do the calculations to make sure. Start by trying these practice questions.
Knowledge Check Quiz
Acids and Bases
Acids and bases are molecules that can influence the pH of a solution. In General Biology it is often convenient to use the Brønsted-Lowry definition of acids and bases. Using this formalism we define:
Acids = molecules that can donate a proton to another molecule (including water to form a hydronium ion)
Bases = molecules that can accept a proton from another molecule (including hydronium ions)
When protons from acidic molecules dissociate from their "parent" they increase the H+ concentration and thereby lower the pH of the solution. By contrast, when a base absorbs a "free" proton from a solution onto the "parent" molecule, the decrease in proton concentration in solution results in a shift to higher pH values.
Generically we can represent acids and bases as follows:
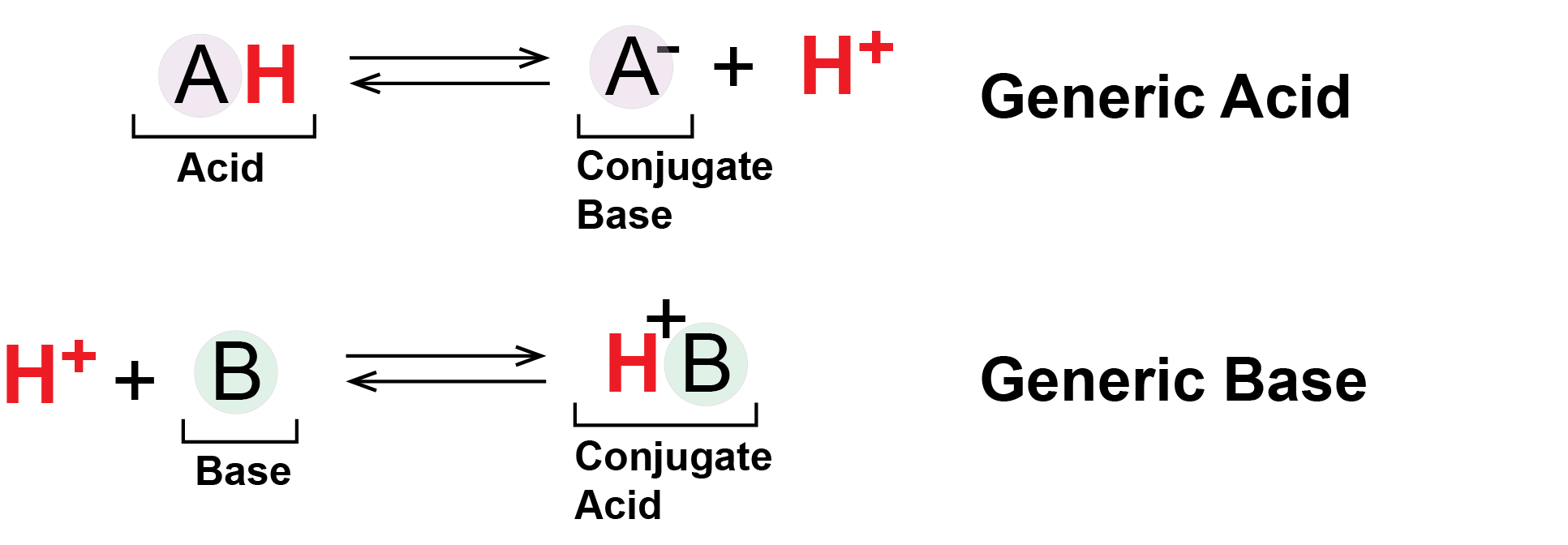
Figure 4: Generic Acids and Bases. This figure shows the behavior of Brønsted-Lowry acids and bases. The acid (A in a light purple circle) starts in a protonated form bound to an H+ ion, drawn as a red H. The acid deprotonates, shedding its H+ into solution or to another molecule. Meanwhile the base (B in a light green circle) begins deprotonated and absorbs a proton (red H+) from solution or other molecule.
Attribution: Marc T. Facciotti
In the figure above, the molecule A- - the deprotonated form of the acid AH - can also be referred to as the conjugate base of the acid AH. Likewise the molecule BH+ - the protonated form of the base B - can be referred to as the conjugate acid of the base B.
We call acids that completely dissociate into A- and H+ ions at equilibrium strong acids. These reactions are characterized by an equilibrium position that lies far to the right (favoring product formation) and their chemical equations are often drawn with a single arrow separating reactants and products. By contrast, acids that do NOT completely dissociate into A- and H+ ions at equilibrium are called weak acids. Depending on the pH, it is common to find both protonated and deprotonated forms of the weak acid (or both the acid and it's conjugate base) in solution at the same time. The chemical equations representing these reactions are therefore usually depicted with double arrows, indicating that the protonation/deprotonation of A-/AH, respectively, is reversible.
Two important examples of weak acids/bases in biology are the carboxyl and amino functional groups. At physiological pH values (around pH = 7) the carboxyl group tends to behave as an acid by donating it's proton to solution or other molecules. Under the same conditions, the amino group tends to act as a base, absorbing protons from solution or other molecules. As we will soon see, these and other protonation/deprotonation reactions play key roles in many biological processes.
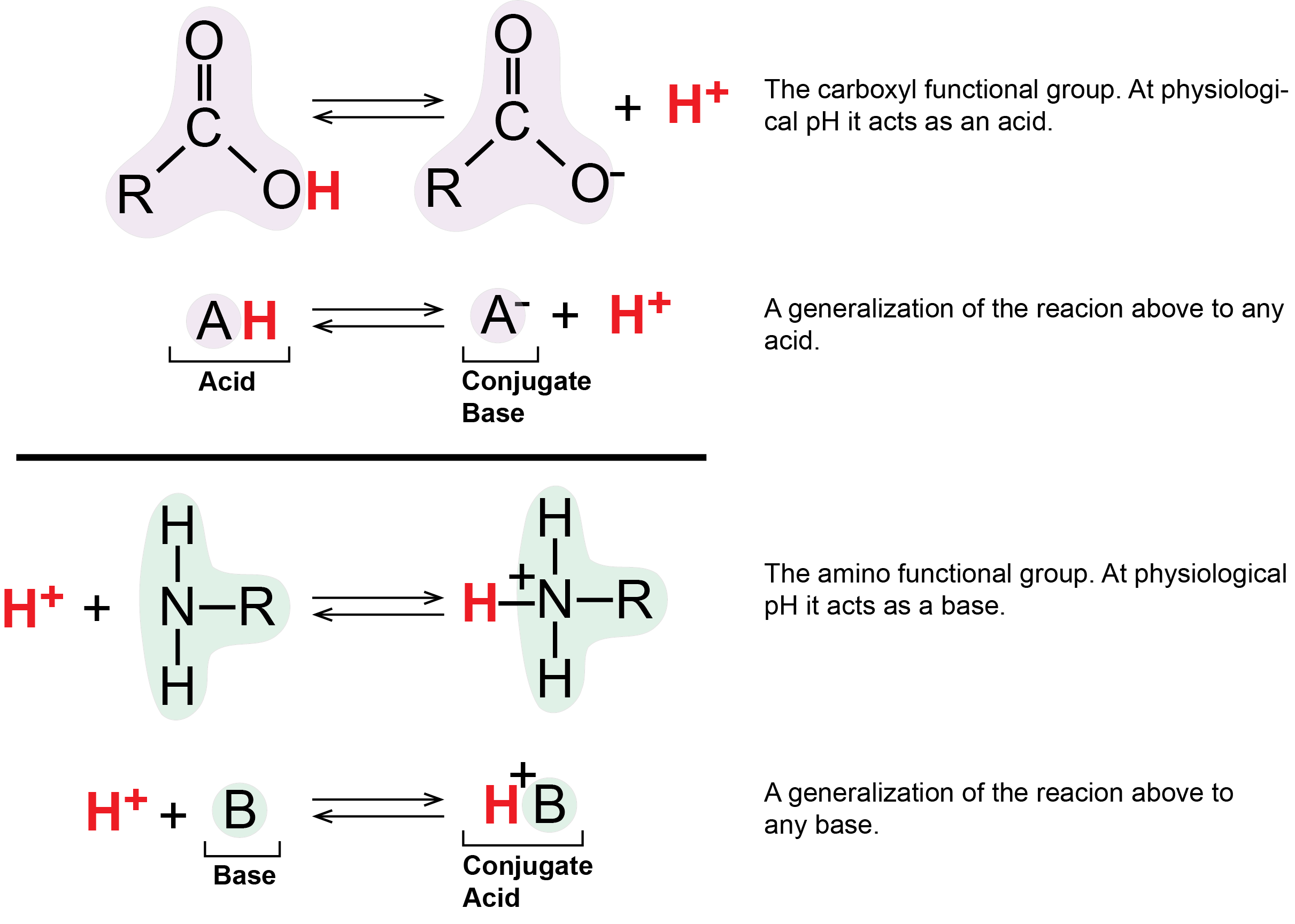
Figure 5: The carboxylic acid group acts as an acid by releasing a proton. This can increase the number of protons in solution and thus decrease the pH. The amino group acts as a base by accepting hydrogen ions, which can decrease the number of hydrogen ions in solutions, thus increasing the pH.
Attribution: Marc T. Facciotti (original work)
Additional pH resources
Here are some additional links on pH and pKa to help learn the material. Note that there is an additional module devoted to pKa.
ChemLibreText Links
- Determining and calculating pH
- pH and pKa
- This has an expanded discussion about pH that is a bit more detailed than how we present the concept.
Khan Academy Links
Simulations
- Acid-base simulation. -------
- ----------embed this in document---------
pKa 
pKa is defined as the negative log10 of the dissociation constant of an acid, its Ka.
pKa = -log10[Ka]
The pKa is a quantitative measure of how readily an acid gives up a proton to a solution and is thus a measure of the "strength" of the acid. Strong acids have a small pKa, weak acids have a larger pKa.
As noted, the carboxylic acid functional group R-COOH is found in many biomolecules. This functional group has a pKa between 2-4 in aqueous solution and is considered a weak acid. At many biologically relevant pH values, the carboxylic acid only partially dissociates into H+ cations and R-COO- anions. In a population of molecules containing the carboxylic acid functional group, it is not uncommon - at biologically relevant pH values - to simultaneously find molecules in both the protonated (R-COOH) and deprotonated (R-COO-) forms. By contrast, HCl (hydrogen chloride), a common strong acid, has a pKa << 0. This means that it will fully dissociate into H+ and Cl- at all biologically relevant pH values. HCl is a strong acid and will almost never be found in the protonated form.
One of the key ways we depict the difference between a strong acid or base and a weak acid or base in chemical equations is the use of a single arrow (strong acid/base) versus a double arrow (weak acid/base). The full dissociation of proton(s) from strong acids at equilibrium means that the likelihood of protons returning to the molecule they dissociated from is very small. The reaction goes mostly one way, hence the single arrow. In weak acids, the likelihood of the forward reaction happening is more similar to the likelihood of the reverse reaction happening. The consequence of this is partial dissociation at equilibrium, hence the double arrow in the chemical equation.

Figure 1. An example of strong acids, weak acids, strong bases, and weak bases in their biologically relevant protonated and deprotonated states. The value of their pKa is shown on the left. Attribution: Marc T. Facciotti.
In General Biology we ask you to relate pH and pKa to each other when discussing the protonation state of a weak acid or weak base in, for example, amino acids. How can we use the information given in this module to answer the question: Will the functional groups on the amino acid Glutamate be protonated or deprotonated at a pH of 2, at a pH of 8, or at a pH of 11?
To answer this kind of question, we need to create a relationship between pH and pKa. The relationship between pKa and pH is mathematically represented by the Henderson-Hasselbach equation shown below, where [A-] represents the deprotonated form of the acid and [HA] represents the protonated form of the acid.
Figure 2. The Henderson-Hasselbach equation
This equation has three core "parts":
1. The pH;
2. The pKa; and
3. log10[A-]/[HA].
Part 1 tells you about the proton concentration. Part 2 tells you about a property of the acid - how likely it is to "give up" its protons to solution. Part 3 tells you about how much of the acid is in its deprotonated form [A-] and in its protonated form [HA]. In most experimental conditions we usually assume that the pKa doesn't change (after all, it's a property of the molecule). So, this equation tells us that the pH and the ratio of deprotonated [A-] and protonated [HA] form of the acid are related to one another. If you are able to independently control the pH by adding more acid or base, you can control the ratio of deprotonated [A-] and protonated [HA] forms of the acid. You can, of course, use the Henderson-Hasselbach equation to solve the problem of knowing the protonation state of Glutamate's functional groups at different pHs. However, we can also develop an intuition about the relationship between these three quantities.
Titrations can help develop an intuitive understanding
Another useful way to develop an intuitive understanding of the relationship between pH, pKa and the protonation states of functional groups is to think about the results of a titration. The titration experiment typically involves the slow, stepwise, and gradual addition of one reagent (e.g. Reagent #1) into a mixture of other molecules (e.g. Solution #1). The experimenter slowly adds Reagent #1 (independent variable) into Solution #1 and makes observations of one or more properties (dependent variables) of the mixture after every step. Depending on the reagents, observations can be things like a change in color, change in viscosity, change in taste, or change in pH. The experimental data is usually plotted in a graph with increasing Reagent #1 on the x-axis and the measured solution property (e.g pH, color, viscosity, etc.) plotted on the y-axis.
Interpreting a Titration Graph
Below is a graph showing the titration of a solution of acetic acid (Solution #1). Acetic acid, the acid found in vinegar, can also be represented by the chemical formula CH3COOH and contains a single carboxyl functional group. In this experiment, the acetic acid is titrated with a base, represented as “OH” (Reagent #1) in the figure. The graph when read from left to right reports the change in pH of the solution when base (OH) is slowly added. Examining the graph, you see three phases of pH change:
(a) Adding between 0 to about 3 equivalents of OH causes a rapid rise of the pH. At the molecular level, this rise can be explained by each additional base equivalent reacting with H3O+ to create two neutral water molecules. This reduces the [H+] and therefore increases the pH. At this pH, the molecule (CH3COOH) has the "strength" to hold onto its H+ ions.
(b) When between 3 and 7 equivalents of OH are added, the pH hardly changes; it stays stable around a value equivalent to the pKa of CH3COOH (4.76). In this zone of the chart, acetic acid molecules start to "let go" of their H+ ions when OH is added. Each OH added “grabs” a proton from solution which is replaced by a proton “released” by CH3COOH molecules in solution. When the pH = pKa (around 5 equivalents of OH-), the Henderson-Hasselbach equation tells us that 50% of the acetic acid molecules in solution are protonated and 50% are deprotonated. As more equivalents of OH are added, more acetic acid molecules become deprotonated until all acetic acid molecules become deprotonated.
(c) Once this happens, at 7 or more equivalents of OH, no more protonated molecules are available to neutralize added OH. Adding more OH therefore starts rapidly raising the pH again.
Building a mental picture of this process can be a powerful tool to help you intuitively solve the problem that started this discussion. If you want to intuit the protonation state of a functional group, with a known pKa, at a given pH, you can start by imagining the situation when the pH = pKa. At this point, you know that the functional group is 50% protonated and 50% deprotonated. Therefore, if the pH in question is below the pKa moving from the pH = pKa to the target pH requires adding protons to the solution. When the solution becomes more acidic, there will be more H+ ions ready to protonate deprotonated functional groups and thus increasing the amount of protonated functional groups. On the titration curve below, this is like starting at the center of the flat portion of the curve and moving to the left. When the target pH is higher than the pKa the solution must become more basic in the shift from the starting pH to the target pH. Here, there will be fewer H+ ions than at the beginning, meaning that the functional groups will start to deprotonate thus increasing the proportion of unprotonated functional groups.
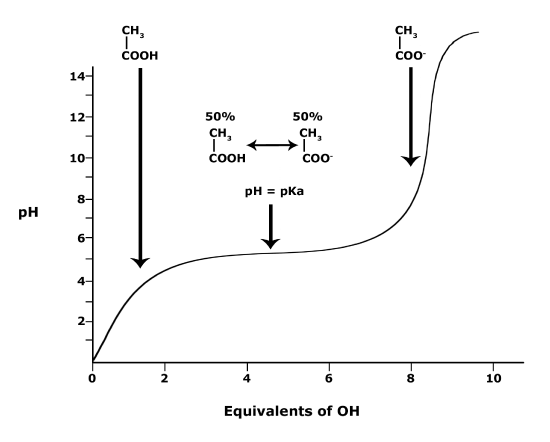
Figure 3. This graph depicts the protonation state of acetic acid as the pH changes. At a pH below the
This movie provides a visual demonstration of the explanation given above.
We close this section by returning to the original question: Will the functional groups on the amino acid glutamate be protonated or deprotonated at a pH of 2, at a pH of 8, at a pH of 11? In the example of acetic acid above, we developed an understanding of how to relate the protonation state of one functional group to its pKa and the pH of the solution its in to the balance/ratio of protonated to deprotonated state for a single functional group.
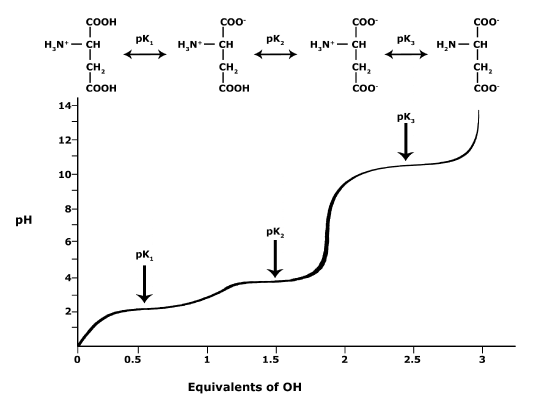
In biology, however, you will often be interested in the behavior of molecules with multiple functional groups, each with their own pKa values. An free amino acid like glutamate has three different functional groups, each with their own pKa. So, answering the question about the protonation state of glutamate at different pH values requires you to assess the protonation state and deprotonation state of each of those functional groups independently. The figure below shows a titration experiment for amino acid glutamate. Like the plot above, it shows the relationship between the pH and pKa for each of the three ionizable functional groups of glutamate. The protonation state of functional group can be evaluated independently at a specified pH value to ultimately determine the protonation state of the whole molecule.
Figure 4. This graph depicts the protonation state of glutamate as the pH changes. At a pH below the pKa for each functional group on the amino acid, the functional group is protonated. At a pH above the pKa for the functional group it is deprotonated. If the pH equals the pKa, the functional group is 50% protonated and 50% deprotonated.
Attribution: Ivy Jose
Interactive Exercise
Quick Reference: pKa and pH Comparison Chart
| pKa | pH |
| pKa = -log10[Ka] | pH= −log10[H+] |
|
|
|
|
|
|
• While pKa depends largely on the physical properties of a molecule, it can also be influenced/changed by the local environment the molecule finds itself in. |
|