10.15: W_2022_Bis2a_Igo_Reading_15
- Page ID
- 74860
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
Learning objectives associated with W_2022_Bis2A_Igo_Reading_15
|
Introduction to Respiration and Electron Transport Chains
General Overview and Points to Keep In Mind
In the next few modules, we learn about the process of respiration and the roles that electron transport chains play in this process. A definition of the word "respiration" that most people are familiar with is "the act of breathing". When we breathe, we bring air, including molecular oxygen, into our lungs from outside of the body. The oxygen then becomes reduced, and waste products, including the reduced oxygen in the form of water, are exhaled. More generically, some reactant comes into the organism and then gets reduced and leaves the body as a waste product.
This generic idea, in a nutshell, can be applied across biology. Note that oxygen need not always be the compound that is brought in, reduced, and dumped as waste. The compounds onto which the electrons that are "dumped" are known as "terminal electron acceptors." The molecules from which the electrons originate vary across biology (so far, we have only looked at one source - the reduced carbon-based molecule glucose).
In between the original electron source and the terminal electron acceptor are a series of biochemical reactions involving at least one redox reaction. These redox reactions harvest energy for the cell by coupling an exergonic redox reaction to an energy-requiring reaction in the cell. In respiration, a special set of enzymes carry out a linked series of redox reactions that ultimately transfer electrons to the terminal electron acceptor.
These "chains" of redox enzymes and electron carriers are called electron transport chains (ETC). In aerobically respiring eukaryotic cells the ETC is composed of four large, multi-protein complexes embedded in the inner mitochondrial membrane and two small diffusible electron carriers shuttling electrons between them. Electrons pass from enzyme to enzyme through a series of redox reactions. These reactions couple exergonic redox reactions to the endergonic transport of hydrogen ions across the inner mitochondrial membrane. This process contributes to the creation of a transmembrane electrochemical gradient. The electrons passing through the ETC gradually lose potential energy until the point the ETC deposits them on the terminal electron acceptor. The cell typically disposes of the reduced terminal electron as waste. When oxygen acts as the final electron acceptor, the free energy difference of this multi-step redox process is ~ -60 kcal/mol when NADH donates electrons or ~ -45 kcal/mol when FADH2 donates.
Note: Oxygen is not the only terminal electron acceptor in nature
Recall, that we use oxygen as an example of only one of many possible terminal electron acceptors that can be found in nature. The free energy differences associated with respiration in anaerobic organisms will be different.
In prior modules we discussed the general concept of redox reactions in biology and introduced the Electron Tower, a tool to help you understand redox chemistry and to estimate the direction and magnitude of potential energy differences for various redox couples. In later modules, substrate level phosphorylation and fermentation were discussed, and we saw that enzymes could directly couple exergonic redox reactions to the endergonic synthesis of ATP.
We hypothesize these processes to be one of the oldest forms of energy production used by cells. In this section, we discuss the next evolutionary advancement in cellular energy metabolism, oxidative phosphorylation. Foremost recall that oxidative phosphorylation does not imply the use of oxygen. Rather, the term oxidative phosphorylation is used because this process of ATP synthesis relies on redox reactions to generate an electrochemical transmembrane potential the cell can then use to do the work of ATP synthesis.
A Quick Overview of Principles Relevant to Electron Transport Chains
An ETC begins with the addition of electrons, donated from NADH, FADH2 or other reduced compounds. These electrons move through a series of electron transporters, enzymes that are embedded in a membrane, or other carriers that undergo redox reactions. The free energy transferred from these exergonic redox reactions is often coupled to the endergonic movement of protons across a membrane. Since the membrane is an effective barrier to charged species, this pumping results in an unequal accumulation of protons on either side of the membrane. This "polarizes" or "charges" the membrane, with a net positive (protons) on one side of the membrane and a negative charge on the other side of the membrane. The separation of charge creates an electrical potential. In addition, the accumulation of protons also causes a pH gradient known as a chemical potential across the membrane. Together these two gradients (electrical and chemical) are called an electro-chemical gradient.
Review: The Electron Tower
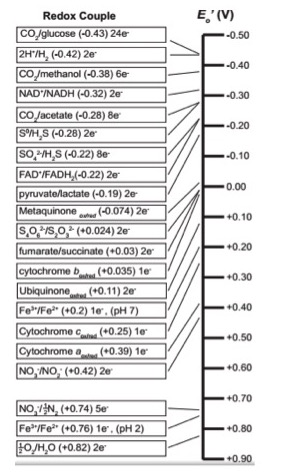
Since redox chemistry is so central to the topic we begin with a quick review of the table of reduction potential - sometimes called the "redox tower" or "electron tower". You may hear your instructors use these terms interchangeably. As we discussed in previous modules, all kinds of compounds can take part in biological redox reactions. Making sense of all of this information and ranking potential redox pairs can be confusing. We have developed a tool to rate redox half reactions based on their reduction potentials or E0' values. Whether a particular compound can act as an electron donor (reductant) or electron acceptor (oxidant) depends on what other compound it is interacting with. The redox tower ranks a variety of common compounds (their half reactions) from most negative E0', compounds that readily get rid of electrons, to the most positive E0', compounds most likely to accept electrons. The tower organizes these half reactions based on the ability of electrons to accept electrons. In addition, in many redox towers, each half reaction is written by convention with the oxidized form on the left followed by the reduced form to its right. The two forms may be either separated by a slash, for example, we write the half reaction for the reduction of NAD+ to NADH: NAD+/NADH + 2e-, or by separate columns. We show an electron tower below.
Figure 1. A common biological "redox tower"
Note
Use the redox tower above as a reference guide to orient you as to the reduction potential of the various compounds in the ETC. Redox reactions may be either exergonic or endergonic depending on the relative redox potentials of the donor and acceptor. Also remember there are many different ways of looking at this conceptually; this type of redox tower is just one way.
Note: Language shortcuts reappear
In the redox table above some entries seem to be written in unconventional ways. For instance Cytochrome cox/red. There only appears to be one form listed. Why? This is another example of language shortcuts (likely because someone was too lazy to write cytochrome twice) that can confuse - particularly to students. We could rewrite the notation above as Cytochrome cox/Cytochrome cred to show that the cytochrome c protein can exist in either and oxidized state Cytochrome cox or reduced state Cytochrome cred.
Review Redox Tower Video
For a short video on how to use the redox tower in redox problems click here. This video was made by Dr. Easlon for Bis2A students.
Using the redox tower: A tool to help understand electron transport chains
By convention, we write the tower half reactions with the oxidized form of the compound on the left and the reduced form on the right. Notice that compounds such as glucose and hydrogen gas are excellent electron donors and have very low reduction potentials E0'. Compounds, such as oxygen and nitrite, whose half reactions have relatively high positive reduction potentials (E0') make we find good electron acceptors at the opposite end of the table.
Example: Menaquinone
Let's look at menaquinoneox/red. This compound sits in the middle of the redox tower with a half-reaction E0' value of -0.074 eV. Menaquinoneox can spontaneously (ΔG<0) accept electrons from reduced forms of compounds with lower half-reaction E0'. Such transfers form menaquinonered and the oxidized form of the original electron donor. In the table above, examples of compounds that could act as electron donors to menaquinone include FADH2, an E0' value of -0.22, or NADH, with an E0' value of -0.32 eV. Remember, the reduced forms are on the right-hand side of the redox pair.
Once menaquinone has been reduced, it can now spontaneously (ΔG<0) donate electrons to any compound with a higher half-reaction E0' value. Electron acceptors include cytochrome box with an E0' value of 0.035 eV; or ubiquinoneox with an E0' of 0.11 eV. Remember that the oxidized forms lie on the left side of the half reaction.
Electron Transport Chains
An electron transport chain, or ETC, is composed of a group of protein complexes in and around a membrane that help energetically couple a series of exergonic/spontaneous red/ox reactions to the endergonic pumping of protons across the membrane to generate an electrochemical gradient. This electrochemical gradient creates a free energy potential that we call a proton motive force whose energetically "downhill" exergonic flow can later be coupled to a variety of cellular processes.
ETC overview
Step 1: Electrons enter the ETC from an electron donor, such as NADH or FADH2, which are generated during a variety of catabolic reactions, including those associated glucose oxidation. Depending on the number and types of electron carriers of the ETC being used by an organism, electrons can enter at a variety of places in the electron transport chain. Entry of electrons at a specific "spot" in the ETC depends upon the respective reduction potentials of the electron donors and acceptors.
Step 2: After the first red/ox reaction, the initial electron donor will become oxidized and the electron acceptor will become reduced. The difference in red/ox potential between the electron acceptor and donor is related to ΔG by the relationship ΔG = -nFΔE, where n = the number of electrons transferred and F = Faraday's constant. The larger a positive ΔE, the more exergonic the red/ox reaction is.
Step 3: If sufficient energy is transferred during an exergonic red/ox step, the electron carrier may couple this negative change in free energy to the endergonic process of transporting a proton from one side of the membrane to the other.
Step 4: After usually multiple red/ox transfers, the electron is delivered to a molecule known as the terminal electron acceptor. With humans, the terminal electron acceptor is oxygen. However, there are many, many, many other possible electron acceptors in nature; see below.
Note: NADH AND FADH2 ARE NOT THE ONLY ELECTRON DONORS
Electrons entering the ETC do not have to come from NADH or FADH2. Many other compounds can serve as electron donors; the only requirements are (1) that there is an enzyme that can oxidize the electron donor and then reduce another compound, and (2) that the ∆E0' is positive (e.g., ΔG<0). Even a small amount of free energy transfers can add up. For example, there are bacteria that use H2 as an electron donor. This is not too difficult to believe because the half reaction 2H+ + 2 e-/H2 has a reduction potential (E0') of -0.42 V. If these electrons are eventually delivered to oxygen, then the ΔE0' of the reaction is 1.24 V, which corresponds to a large negative ΔG (-ΔG). Alternatively, there are some bacteria that can oxidize iron, Fe2+ at pH 7 to Fe3+ with a reduction potential (E0') of + 0.2 V. These bacteria use oxygen as their terminal electron acceptor, and, in this case, the ΔE0' of the reaction is approximately 0.62 V. This still produces a -ΔG. The bottom line is that, depending on the electron donor and acceptor that the organism uses, a little or a lot of energy can be transferred and used by the cell per electrons donated to the electron transport chain.
What are the complexes of the ETC?
ETCs comprise a series (at least one) of membrane-associated red/ox proteins or (some are integral) protein complexes (complex = more than one protein arranged in a quaternary structure) that move electrons from a donor source, such as NADH, to a final terminal electron acceptor, such as oxygen. This specific donor/terminal acceptor pair is the primary one used in human mitochondria. Each electron transfer in the ETC requires a reduced substrate as an electron donor and an oxidized substrate as the electron acceptor. In most cases, the electron acceptor is a member of the enzyme complex itself. Once the complex is reduced, the complex can serve as an electron donor for the next reaction.
How do ETC complexes transfer electrons?
As previously mentioned, the ETC is composed of a series of protein complexes that undergo a series of linked red/ox reactions. These complexes are in fact multi-protein enzyme complexes referred to as oxidoreductases or simply, reductases. The one exception to this naming convention is the terminal complex in aerobic respiration that uses molecular oxygen as the terminal electron acceptor. That enzyme complex is referred to as an oxidase. Red/ox reactions in these complexes are typically carried out by a non-protein moiety called a prosthetic group. The prosthetic groups are directly involved in the red/ox reactions being catalyzed by their associated oxidoreductases. In general, these prosthetic groups can be divided into two general types: those that carry both electrons and protons and those that only carry electrons.
Note:
This use of prosthetic groups by members of ETC is true for all of the electron carriers with the exception of quinones, which are a class of lipids that can directly be reduced or oxidized by the oxidoreductases. Both the Quinone(red) and the Quinone(ox) forms of these lipids are soluble within the membrane and can move from complex to complex to shuttle electrons.
The electron and proton carriers
- Flavoproteins (Fp), these proteins contain an organic prosthetic group called a flavin, which is the actual moiety that undergoes the oxidation/reduction reaction. FADH2 is an example of an Fp.
- Quinones are a family of lipids, which means they are soluble within the membrane.
- We also note that we consider NADH and NADPH electron (2e-) and proton (2 H+) carriers.
Electron carriers
- Cytochromes are proteins that contain a heme prosthetic group. The heme can carry a single electron.
- Iron-Sulfur proteins contain a nonheme iron-sulfur cluster that can carry an electron. We often abbreviate the prosthetic group as Fe-S
Aerobic versus anaerobic respiration
We humans use oxygen as the terminal electron acceptor for the ETCs in our cells. This is also the case for many of the organisms we intentionally and frequently interact with (e.g. our classmates, pets, food animals, etc). We breathe in oxygen; Our cells take it up and transport it into the mitochondria, where it becomes the final acceptor of electrons from our electron transport chains. We call the process where oxygen is the terminal electron acceptor aerobic respiration.
While we may use oxygen as the terminal electron acceptor for our respiratory chains, this is not the only mode of respiration on the planet. The more general processes of respiration evolved when oxygen was not a major component of the atmosphere. As a result, many organisms can use a variety of compounds, including nitrate (NO3-), nitrite (NO2-), even iron (Fe3+) as terminal electron acceptors. When oxygen is NOT the terminal electron acceptor, we refer the process to as anaerobic respiration. Therefore, respiration or oxidative phosphorylation does not require oxygen at all; It requires a compound with a high enough reduction potential to act as a terminal electron acceptor, accepting electrons from one complex within the ETC.
The ability of some organisms to vary their terminal electron acceptor provides metabolic flexibility and can ensure better survival if any given terminal acceptor is in limited supply. Think about this: in the absence of oxygen, we die; but other organisms can use a different terminal electron acceptor when conditions change to survive.
Possible NB Discussion  Point
Point
Nature has figured out how to use different molecules as terminal electron acceptors of ETCs. Yet humans seem limited to using only oxygen. Can you offer any hypotheses why humans have not evolved to use multiple different terminal electron acceptors? Why do you think it might be advantageous for an organism to use oxygen as a sole terminal electron acceptor?
A generic example: A simple, two-complex ETC
The figure below depicts a generic electron transport chain, composed of two integral membrane complexes; Complex I(ox) and Complex II(ox). A reduced electron donor, designated DH (such as NADH or FADH2) reduces Complex I(ox), giving rise to the oxidized form D (such as NAD+ or FAD+). Simultaneously, a prosthetic group within Complex I is now reduced (accepts the electrons). In this example, the red/ox reaction is exergonic and the free energy difference is coupled by the enzymes in Complex I to the endergonic translocation of a proton from one side of the membrane to the other. The net result is that one surface of the membrane becomes more negatively charged, because of an excess of hydroxyl ions (OH-), and the other side becomes positively charged because of an increase in protons on the other side. Complex I(red) can now reduce a mobile electron carrier Q, which will then move through the membrane and transfer the electron(s) to the prosthetic group of Complex II(red). Electrons pass from Complex I to Q then from Q to Complex II via thermodynamically spontaneous red/ox reactions, regenerating Complex I(ox), which can repeat the previous process. Complex II(red) then reduces A, the terminal electron acceptor to regenerate Complex II(ox) and create the reduced form of the terminal electron acceptor, AH. In this specific example, Complex II can also translocate a proton during the process. If A is molecular oxygen, AH represents water and the process would be considered being a model of an aerobic ETC. If A is nitrate, NO3-, then AH represents NO2- (nitrite) and this would be an example of an anaerobic ETC.
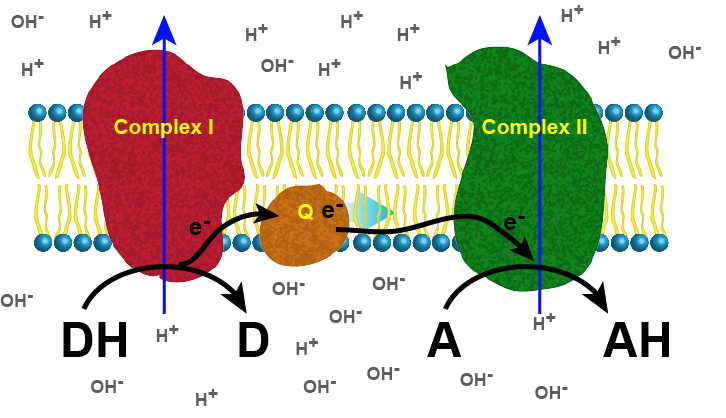
Figure 1. Generic 2 complex electron transport chain. In the figure, DH is the electron donor (donor reduced), and D is the donor oxidized. A is the oxidized terminal electron acceptor, and AH is the final product, the reduced form of the acceptor. As DH is oxidized to D, protons are translocated across the membrane, leaving an excess of hydroxyl ions (negatively charged) on one side of the membrane and protons (positively charged) on the other side of the membrane. The same reaction occurs in Complex II as the terminal electron acceptor is reduced to AH. Attribution: Marc T. Facciotti (original work)
Detailed look at aerobic respiration
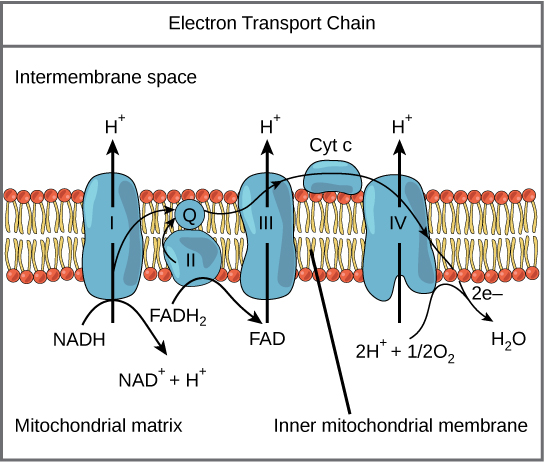
The eukaryotic mitochondria have evolved a very efficient ETC. There are four complexes composed of proteins, labeled I through IV depicted in the figure below. The aggregation of these four complexes, together with associated mobile, accessory electron carriers, is called an electron transport chain. This electron transport chain is present in multiple copies in the inner mitochondrial membrane of eukaryotes.
Figure 2. The electron transport chain is a series of electron transporters embedded in the inner mitochondrial membrane that shuttles electrons from NADH and FADH2 to molecular oxygen. In the process, protons are pumped from the mitochondrial matrix to the intermembrane space, and oxygen is reduced to form water.
Complex I
To start, NADH delivers two electrons to the first protein complex. This complex, labeled I in Figure 2, includes flavin mononucleotide (FMN) and iron-sulfur (Fe-S)-containing proteins. FMN, which is derived from vitamin B2, also called riboflavin, is one of several prosthetic groups or cofactors in the electron transport chain. Prosthetic groups are organic or inorganic, nonpeptide molecules bound to a protein that facilitate its function; prosthetic groups include coenzymes, which are the prosthetic groups of enzymes. We also call the enzyme in Complex I NADH dehydrogenase. This protein complex contains 45 individual polypeptide chains. Complex I can pump four hydrogen ions across the membrane from the matrix into the intermembrane space helping to generate and maintain a hydrogen ion gradient between the two compartments separated by the inner mitochondrial membrane.
Q and Complex II
Complex II directly receives FADH2, which does not pass through Complex I. The compound connecting the first and second complexes to the third is ubiquinone (Q). The Q molecule is lipid soluble and freely moves through the hydrophobic core of the membrane. Once reduced, (QH2), ubiquinone delivers its electrons to the next complex in the electron transport chain. Q receives the electrons derived from NADH from Complex I and the electrons derived from FADH2 from Complex II, succinate dehydrogenase. Since these electrons bypass and thus do not energize the proton pump in the first complex, fewer ATP molecules are made from the FADH2 electrons. As we will see in the following section, the number of ATP molecules ultimately obtained is directly proportional to the number of protons pumped across the inner mitochondrial membrane.
Complex III
The third complex is composed of cytochrome b, another Fe-S protein, Rieske center (2Fe-2S center), and cytochrome c proteins; we also call this complex cytochrome oxidoreductase. Cytochrome proteins have a prosthetic group of heme. The heme molecule is like the heme in hemoglobin, but it carries electrons, not oxygen. As a result, the iron ion at its core is reduced and oxidized as it passes the electrons, fluctuating between different oxidation states: Fe2+ (reduced) and Fe3+ (oxidized). The heme molecules in the cytochromes have slightly different characteristics because of the effects of the different proteins binding them, giving slightly different characteristics to each complex. Complex III pumps protons through the membrane and passes its electrons to cytochrome c for transport to the fourth complex of proteins and enzymes (cytochrome c is the acceptor of electrons from Q; however, whereas Q carries pairs of electrons, cytochrome c can accept only one at a time).
Complex IV
The fourth complex is composed of cytochrome proteins c, a, and a3. This complex contains two heme groups (one in each of the two Cytochromes, a, and a3) and three copper ions (a pair of CuA and one CuB in Cytochrome a3). The cytochromes hold an oxygen molecule tightly between the iron and copper ions until it completely reduces the oxygen. The reduced oxygen then picks up two hydrogen ions from the surrounding medium to make water (H2O). The removal of the hydrogen ions from the system contributes to the ion gradient used in the process of chemiosmosis.
Chemiosmosis
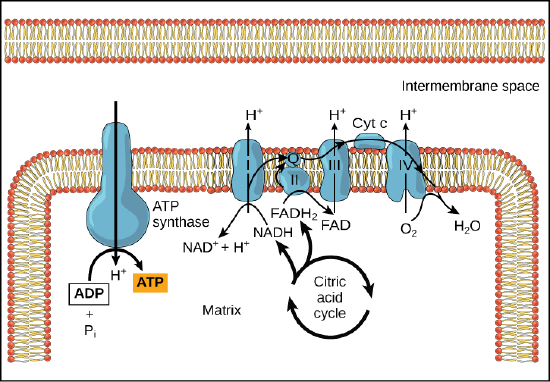
In chemiosmosis, the free energy from the series of red/ox reactions just described is used to pump protons across the membrane. The uneven distribution of H+ ions across the membrane establishes both concentration and electrical gradients (thus, an electrochemical gradient), owing to the proton's positive charge and their aggregation on one side of the membrane.
If the membrane were open to diffusion by protons, the ions would tend to diffuse back across into the matrix, driven by their electrochemical gradient. Ions, however, cannot diffuse through the nonpolar regions of phospholipid membranes without the aid of ion channels. Similarly, protons in the intermembrane space can only traverse the inner mitochondrial membrane through an integral membrane protein called ATP synthase (depicted below). This complex protein acts as a tiny generator, turned by transfer of energy mediated by protons moving down their electrochemical gradient. The movement of this molecular machine (enzyme) serves to lower the activation energy of reaction and couples the exergonic transfer of energy associated with the movement of protons down their electrochemical gradient to the endergonic addition of a phosphate to ADP, forming ATP.
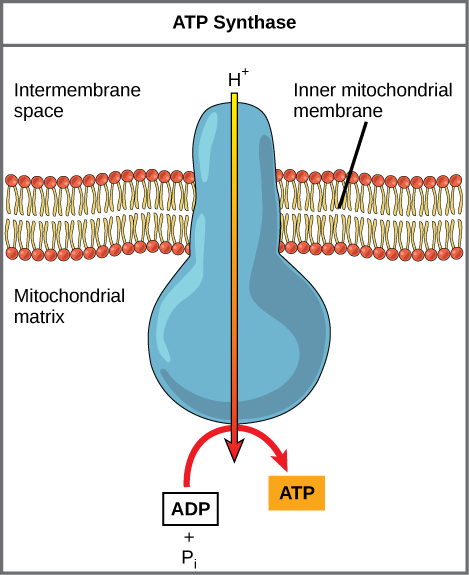
Figure 3. ATP synthase is a complex, molecular machine that uses a proton (H+) gradient to form ATP from ADP and inorganic phosphate (Pi). Credit: modification of work by Klaus Hoffmeier
Possible NB Discussion  Point
Point
Cyanide inhibits cytochrome c oxidase, a component of the electron transport chain. If cyanide poisoning occurs, would you expect the pH of the intermembrane space to increase or decrease? What effect would cyanide have on ATP synthesis? How would this affect the rates of reactions in glycolysis and the TCA cycle?
In healthy cells, chemiosmosis (depicted below) is used to generate 90 percent of the ATP made during aerobic glucose catabolism; it is also the method used in the light reactions of photosynthesis to harness the energy of sunlight in the process of photophosphorylation. Recall that the production of ATP using the process of chemiosmosis in mitochondria is called oxidative phosphorylation and that a similar process can occur in the membranes of bacterial and archaeal cells. The overall result of these reactions is the production of ATP from the energy of the electrons removed originally from a reduced organic molecule like glucose. In the aerobic example, these electrons ultimately reduce oxygen and create water.
Figure 4. In oxidative phosphorylation, the pH gradient formed by the electron transport chain is used by ATP synthase to form ATP in a Gram-bacteria.
Helpful link: How ATP is made from ATP synthase
A Hypothesis for How ETCs May Have Evolved
A proposed link between SLP/fermentation and the evolution of ETCs:
In a previous discussion of energy metabolism, we explored substrate level phosphorylation (SLP) and fermentation reactions. While SLP and fermentation together are perfectly good ways to harvest energy, one byproduct of these reactions is the acidification of the cell. Early cells that used these modes of energy harvested therefore needed to co-evolve mechanisms that helped remove protons accumulated from SLP and fermentation from the cytosol (interior of the cell). One solution to the "proton problem" may have been the evolution of the F0F1-ATPase, a multi-subunit enzyme that translocated protons from the inside of the cell to the outside of the cell by hydrolyzing ATP (see the figure below). This arrangement works as long as small reduced organic molecules are abundant and freely available to generate ATP can through SLP that can "fuel" the business of the cell. However, as these biological processes continue, the small reduced organic molecules will become depleted. The resulting scarcity of fuel, therefore, puts a demand on cells to find alternative mechanisms to harness energy and/or to become more efficient.
In the scheme proposed above, one potential source of "wasted ATP" is its use in the removal of protons from the cell's cytosol; organisms that could find other mechanisms to expel accumulating protons while still preserving ATP could have a selective advantage. We hypothesize that this selective evolutionary pressure potentially led to the evolution of the first membrane-bound proteins that used red/ox reactions as their energy source (depicted in a second picture) to pump out the accumulating protons. Enzymes and enzyme complexes with these properties exist today in the as electron transport complexes like Complex I, the NADH dehydrogenase.
Figure 1. Proposed evolution of an ATP dependent proton translocator
Figure 2. As small reduced organic molecules become limited, organisms that can find alternative mechanisms to remove protons from the cytosol may have an a selective advantage. The evolution of a proton translocator that uses red/ox reactions rather than ATP hydrolysis could substitute for the ATPase.
Continuing with this line of logic, if organisms evolved that could now use red/ox reactions to translocate protons across the membrane they would create an electrochemical gradient, separating both charge (positive on the outside and negative on the inside, creating an electrical potential) and pH (low pH outside, higher pH inside). With excess protons on the outside of the cell membrane, and the F0F1-ATPase no longer consuming ATP to translocate protons, we hypothesize that the electrochemical gradient could then power the F0F1-ATPase "backwards" — that is, to form or produce ATP by using the energy in the charge/pH gradients set up by the red/ox pumps (as depicted below). We call this arrangement an electron transport chain (ETC).
Figure 3. The evolution of the ETC; the combination of the red/ox driven proton translocators coupled to the production of ATP by the F0F1-ATPase.
Note: Extended reading on the evolution of electron transport chains
If you're interested in the the evolution of electron transport chains, check out this more in-depth discussion of the topic at NCBI.
Possible NB Discussion  Point
Point
Dinitrophenol (DNP) is a small chemical that serves to uncouple the flow of protons across the inner mitochondrial membrane to the ATP synthase, making the membrane leaky to protons. People used it until 1938 as a weight-loss drug. What effect would you expect DNP to have on the difference in pH across both sides of the inner mitochondrial membrane? Why do you think this might be an effective weight-loss drug? Why might it be dangerous? Can you think of any scenarios where it is non-harmful, or even beneficial, to uncouple proton flow with ATP synthase?
An introduction to metabolic regulation
Metabolic pathways
We can describe cellular metabolic pathways as sets of interconnected enzyme-catalyzed chemical reactions. Together these pathways form complex networks that collectively guide the flow of molecules and energy throughout a cell by breaking down, building, and rearranging the atoms and molecules of nature into their many different forms.
The collective activity of metabolic pathways allows organisms to eat food (e.g. atoms and molecules) from their environments and to convert that food at a molecular level into the specific forms of matter the organism needs to support life. Metabolic pathways also allow organisms to harvest energy from the environment and to move that energy to places in their cells that need energy to get work done.
Understanding how metabolic pathways help biological systems reorganize matter and energy is therefore fundamental to our understanding of biology from the smallest cellular scale to the functioning of connected global ecosystems and their interactions with the non-living environment.
Metabolic decision making, sensors and switches
Many living systems have complex metabolic pathways that enable them to make and process many different types of molecules. While these complex pathways likely provide an organism with selective advantage not all pathways need to be functioning at the same levels all of the time.
If, for instance, a unicellular organism is able to find and eat plenty of the amino acid tryptophan from its environment, the organism’s metabolic pathways that synthesize tryptophan do not need to be highly active. Running metabolic pathways that don’t need to be active wastes both the atoms that pass through the pathways and the energy used in the creation of molecules that aren’t needed at a particular time. Moreover, since most living systems do not exist in resource-rich environments, atoms and energy must be carefully distributed to cellular functions that need them most, only when they are needed. Resources can then be converted into other forms when they are needed elsewhere in the cell.
To be competitive, the cell must be frugal with resources and thus control how and when different metabolic pathways are active. Controlling the flow of materials through metabolic pathways requires mechanisms for decision making - we use the anthropomorphic word “decision” to imply that the cell seemingly knows how to make choices, not to suggest that it has a brain. For example, the cell in the example above needs a way to know that tryptophan is in abundance and then to use that information to switch off the biosynthetic pathway for tryptophan biosynthesis. Concomitantly, the cell can choose to use the metabolic precursors for tryptophan biosynthesis for another purpose, activating enzymes in other pathways that can use those same precursor molecules. This kind of sensing and control is required to simultaneously coordinate the flow of biomolecules and energy throughout all of cellular metabolism. This means that there must be molecular sensors and switches for controlling metabolism distributed throughout the cell.

Figure 1. Overview of the major connections between core metabolic pathways in eukaryotes.
Attribution:Chakazul / CC BY-SA (https://creativecommons.org/licenses/by-sa/4.0) <https://upload.wikimedia.org/wikipedia/commons/6/6e/Metabolic_Metro_Map.svg>
Our daily lives are full of similar experiences and concepts that can inform how we think about metabolic control in biology. Our homes have all sorts of sensors and switches for managing energy. We have light switches in every room to control the flow of electricity and conversion of energy through light bulbs. Our stoves have switches on them to control energy use on each burner. Thermostats in the home sense temperature and use that information to adjust the activity of our air conditioners and/or heaters. Some of these switches are bistable, they turn things fully “on” or “off” (e.g. most light switches). Other switches are continuous (e.g. dial switches on a stove, the volume knob on the radio). They allow the user to control energy use along a gradient.
The hierarchical nature of regulatory systems
Another useful concept to draw from looking at how we manage energy in our homes is to note the hierarchical nature of the control systems we have engineered for our living spaces. Nearly all homes/apartments have circuit breakers that can help control the flow of power from an energy provider into the home. In a circuit breaker box one usually finds a master switch that can turn power on or off for the entire home. One can usually also find switches that control power to individual rooms or parts of the house (e.g. the kitchen, the bathrooms). Within each room, light switches mounted on the wall typically allow the resident to control energy flow to all or just some of the outlets in the wall. Finally, each device plugged into the wall has its own “on”/“off” switch. Many devices, like a radio, have additional switches on them that allow individualized energy use management. As we develop our understanding of regulation in biology, we will see that Nature has also evolved conceptually similar hierarchical control structures (though typically more interconnected) to help manage metabolism and various other cellular functions. Some signaling molecules target and regulate very specific control points - a single enzyme. Other molecular signals target many control points simultaneously (many enzymes can be coordinately regulated). The interplay between specific control signals and broader control signals creates the ability to respond to big changes quickly and also to fine tune responses in the cell in a very detailed way.
Figure 2. A schematic of a household wiring scheme showing hierarchical control of power to rooms, lights and devices. The master switch can turn off all power. Sub-switches can control power to individual rooms. Meanwhile, room-level switches can control power to parts of the room. Finally, switches on devices can control power at an individual device level. This serves as an analogy for some features of metabolic and cellular regulation.
Attribution: Original work - Marc T. Facciotti
The Who, What, When, Where, Why and How of Regulation
We structure the rest of this discussion of metabolic regulation by using a formalism often associated with journalism. We examine answers to the questions who, what, when, where, why and how <https://en.wikipedia.org/wiki/Five_Ws>.
Why regulate?
To some degree, we have made the argument about why regulation is necessary above. Largely, regulation in metabolism is involved in making decisions about how to best manage limited resources - the atoms that make up biomolecules, the biomolecules themselves, and the energy needed to build new molecules and/or carry out cellular functions. Good decision-making leads to more efficient resource utilization and by consequence we suspect higher evolutionary fitness.
Who to regulate?
When we consider the regulation of metabolism and metabolic pathways, the key control points are the enzymes that catalyze individual reactions. In the simplest terms, increasing the abundance of a specific enzyme and/or increasing its activity (how fast it catalyzes a reaction) can increase the flow of molecules through that step of a pathway. By contrast, decreasing the abundance of an enzyme and/or lowering its activity will reduce the amount of material flowing through that step in a pathway.
Where should regulation be exercised?
If enzymes and their relative abundance are good targets of regulation we can now ask whether some steps in metabolic pathways are more important to regulate than others. The answer to this question is yes and can be largely summarized by finding enzymes that belong to one or more of three classes: (a) enzymes that catalyze steps at branch points in a pathway; (b) enzymes that catalyze rate-limiting reactions; and (c) enzymes that catalyze so-called irreversible reactions (i.e. reaction with a large negative ∆G). We’ll examine the logic behind each.
Enzymes that catalyze reactions at branch points: Branch points in metabolic reactions are points in a metabolic pathway in which a compound can be used as a substrate in two or more different biochemical reactions. The cell must “decide” which of the two or more pathways to direct the biomolecule to. This is no different than the everyday experience we each time we encounter an intersection in the road we’re traveling on - we must decide whether to continue straight or turn down a different path. We can’t do both at the same time. Another everyday example might be in the construction of a water distribution pipeline in a garden with two or more planting beds. Depending on what is planted in each bed the gardener may wish to tune the flow of water to each bed differently and would do so by opening and closing valves at the branch points of the watering system.
Enzymes that catalyze rate-limiting steps in a pathway: A rate-limiting step in a metabolic pathway is defined as the reaction that determines how fast the overall pathway overall can convert input into output. This is almost always the slowest step in the pathway. Regulating this step by either slowing or speeding flow of metabolites through it can change the flow for the entire pathway. By analogy, you can think of a 5-lane highway that needs to constrict to 3 lanes over a bridge. While the highway may widen back up to 5 lanes after the bridge, the maximum rate of traffic flow before the bridge and after the bridge is limited by how many cars can get across the bridge at any one time. Narrowing or widening the bridge will have a major impact on traffic flow both before and after.
Enzymes that catalyze irreversible reactions: Reactions that have a large negative ∆G are often called irreversible reactions. These reactions are also often considered to be “commitment” steps in a biochemical pathway because it is difficult to reverse the reaction once it is done. Typically, different enzymes and external energy sources are required to run the reaction in the endergonic direction. We can also draw upon an analogy here to understand why these are important decision points by thinking about decisions in life that are hard to take back once they are made. Consider, for instance, the act of buying a used car or other equivalent item of value that you might need, or care to have. Few people would consider buying such an item sight unseen and without asking questions, doing some research, and maybe even getting a professional opinion on the condition of the car or other item. If the car/item is still functional and well-maintained the purchase could work out fantastically well. On the other hand, if the car/item has some defects or in need of repair, the purchase may not end well at all. The decision to buy the car/item must be carefully considered BEFORE the purchase. After you hand over the money there is typically no opportunity to changing your mind – the sale if final - and you will need to live with the consequences of that decision, good or bad. The same idea applies to reactions with large negative ∆G. Once the decision has been made to catalyze the reaction, that decision cannot be easily reversed. Therefore, the cell must evolve mechanisms that help make these decisions carefully on the basis of good information.
In many metabolic pathways, particularly those that are as interconnected to other pathways as glycolysis, the picture is - of course - more complicated as each these three types of control points are each used for regulation and it is too simplistic to simply state that there is a single point in the pathway that controls flow. Rather, it is the combination and interplay of regulatory sites and regulatory mechanisms that ultimately determines flow into, through, and out of the pathway.
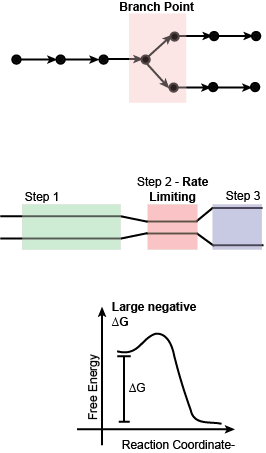
Figure 3. Schematic of three important types of steps in metabolic pathways that are key targets of regulation.
Attribution: Original work - Marc T. Facciotti
When to regulate?
A cell is always regulating the flow of molecules through its metabolic pathways. Understanding when to change the regulation of different metabolic pathways is the critical issue. When does the organism start to run glycolysis? When does it ramp up nucleotide biosynthesis and turn down amino acid biosynthesis? The short answer is that pathways are up-regulated when they are needed and down-regulated when they aren’t. But how does the organism know when something is needed and something else isn’t? The answer to this question is a bit more involved than what we can discuss in the time and space provided here. However, we can intuit that whatever these processes are, they must involve the sensing of environmental and/or cellular information. Something must be measured.
What to measure?
In the context of metabolic pathways, what information could be measured to help a cell make decisions about whether to up- or down-regulate flow through a metabolic pathway? Since metabolic pathways are in “the business” of consuming input molecules and converting them into some other product, it might be reasonable to expect that knowing whether there is enough product in the cell already (in which case the pathway doesn’t need to run) or whether there is enough of the original substrate around to feed the pathway could be useful information to know for regulation. Other cellular information of relevance to metabolism that might be useful to know about when deciding on whether to up- or down-regulate a pathway is the general level of usable energy (e.g. the balance between levels of ATP, ADP, and AMP) and the availability of reduced and oxidized electron carriers (e.g. the balance between NADH and NAD+). Clearly, in some cases the levels and balance between other molecules will also be important.
How can the cell use molecular information to make metabolic decisions?
Above we suggested some rationale about the regulation of metabolic pathways. We proposed that metabolic pathways must be regulated to manage cellular resources (why) and that this regulation should happen in response to changing cellular needs (when). We propose that regulation happens when branch points, irreversible reactions, and rate-limiting steps are found (where). Furthermore, we suggest that regulation happen by controlling the abundance and/or activity of the enzymes that catalyze reactions in a pathway (who). Finally, we posit that knowing the abundance of pathway inputs and products as well as general indicators of cellular energy and redox stores (what) is generally useful for cellular decision-making.
This leaves us to answer the final question. How? How can a cell use abundance of cellular molecules to inform enzymes catalyzing key steps in metabolic pathways about whether they are needed more or less when cellular conditions change? The complete answer to this question is, like seemingly everything else in biology, multifaceted, involving different targets of regulation (e.g. the genes encoding an enzyme to the enzyme itself) and different mechanisms. Here we briefly discuss one mechanism that links back to an earlier lesson on proteins; allosteric regulation.
Allosteric regulation involves the binding of molecules to allosteric sites on an enzyme that are by definition not in the active site. This binding alters the protein structure and can cause in different instances both either up or down regulation of enzyme activity. Often, enzymes that are the subject of allosteric regulation can be influenced by more than one ligand. By binding different phosphorylation states of ATP (i.e. AMP, ADP, and ATP) at allosteric sites enzymes can measure the ratios of ATP to its other forms and thus assess the energy “status” of the cell.
Examples of metabolic regulation by allosteric binding
Reactions with large negative ∆G
An early intermediate step of glycolysis, the phosphorylation of fructose-6-phosphate by ATP to yield fructose-1,6-bisphosphate and ADP, has an approximate ∆G of -19kJ/mol under cellular conditions. This is one of three largest, by comparison to all other reactions in the pathway, drops in free energy in the glycolytic pathway. In mammalian systems, the enzyme catalyzing this reaction, phosphofructokinase, is both positively and negatively regulated by multiple small molecules. ATP, citrate, and phosphoenolpyruvate (PEP) have been found to bind allosteric sites on the enzyme and lead to a lowering of enzyme activity. AMP, ADP, and fructose-2,6-bisphosphate (a product created by another enzyme) each increase phosphofructokinase activity. A number of other small molecules have also been found to influence enzyme activity in the test tube, but these are typically considered to play an insignificant role in the living system.
Given the central role of the glycolytic pathway in both energy harvesting and the creation of key precursors for other metabolic pathways, it is perhaps not surprising to have this enzyme’s activity influenced by a measure of the energy status of the cell (the ATP/AMP ratio) and by molecules in connected pathways (PEP and citrate). It is perhaps easiest to understand that when the enzyme senses low energy levels in the cell, when the ATP/AMP ratio is low, that the enzyme committing sugar to enter the energy extraction phase of glycolysis should become more active and that the converse should be true when ATP is abundant.

Figure 4. The enzyme phosphofructokinase is regulated allosterically both positively and negatively by numerous small molecules indicators of cellular energy state and of key concentrations of pathway intermediates. Attribution: Original work - Marc T. Facciotti
Branch Points
An example of branch point regulation can be found in the pathways leading to the biosynthesis of aromatic amino acids (i.e. Phenylalanine, Tyrosine, and Tryptophan). The synthesis of each of these three amino acids begins with the production of the compound Chorismate. This compound can then be taken down two independent pathways. The first leads to Phenylalanine and Tyrosine biosynthesis while the second leads to Tryptophan biosynthesis. The enzyme catalyzing the first step in the path towards Phenylalanine and Tyrosine, Chrosimate mutase, is negatively regulated by the two products of the pathway and activated by Tryptophan, the product of the second pathway. Meanwhile, the enzyme Anthranilate Synthase is negatively regulated by the final product of its pathway, Tryptophan. The feedback of end-product levels on the enzymes responsible for catalyzing the synthesis of these three amino acids allow the cell to decide how to best utilize the stock of Chorismate and make that decision at the one of the core branch points for its use.
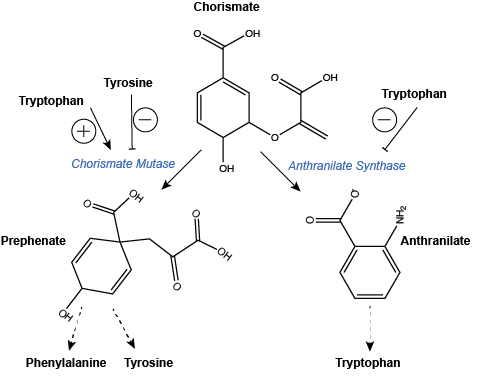
Figure 5: Regulation of branchpoint enzymes in the biosynthesis of aromatic amino acids. Dashed arrows indicate one or more steps are still required to create product of the pathway and these are not drawn explicitly. Note that this is also an example of pathway products feeding back to regulate upstream enzyme activity.
Attribution: Original work - Marc T. Facciotti