Spring_2024_Bis2A_Igo_Lecture_Reading_08
- Page ID
- 133381
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Learning Objectives Associated with Spring_2024_Bis2A_Igo_Lecture_08CS.5 Discuss the advantages and trade-offs associated with simple diffusion, passive diffusion, facilitated diffusion, and active transport. MS.16 Predict the properties of the R-groups on amino acids located in different locations within transmembrane transporters. CS.8 Understand the role of the plasma membrane in maintaining chemical and electrical gradients. GC.42 Predict whether two reactions can be theoretically productively coupled by interpreting tables of standard Gibbs enthalpy (energy) (From Lecture Reading 6). GC.50 Describe why an enzyme is critical for coupling an exergonic reaction to an endergonic reaction by applying the concepts from the chemistry and thermodynamics lectures (From Lecture Reading 6). NOTE: MANY OF THE LEARNING OBJECTIVES BELOW WILL BE COVERED IN THE WEEK 3 DISCUSSION SECTION GC.28 Identify biological redox reactions involving common electron carriers. GC.29 Given a redox reaction, identify the reducing agent, oxidizing agent, molecule that becomes oxidized, and the reduced species. Identify which species the electron(s) "starts" in, and to which species it "goes." GC.30 Write a composite chemical equation when given two redox half-reactions. GC.31 Calculate the ΔE0’ for a given redox reaction using the equation ΔE0’ = E0’(oxidant) - E0’(reductant) GC.43 Qualitatively relate the difference in redox potentials with a corresponding delta of Gibbs enthalpy (energy). GC.26 Predict whether a directional transfer of electrons between two chemical species is endergonic or exergonic by applying the concept of redox potential to provided data. ME.2 Identify NAD+ from its molecular structure and identify the functional group involved in its function as an oxidizing or reducing agent. ME.1 Tell an energy story for a redox reaction that utilizes the electron carrier NAD+/NADH as the second substrate in the simple, generic reaction scheme: AH + NAD+ -> A + NADH.
|
 Energetics of transport
Energetics of transport
All substances that move through the membrane do so by one of two general methods, which are categorized based on whether the transport process is exergonic or endergonic. Passive transport is the exergonic movement of substances across the membrane. In contrast, active transport is the endergonic movement of substances across the membrane that is coupled to an exergonic reaction.
Passive Transport
Passive transport does not require the cell to expend energy. In passive transport, substances move from an area of higher concentration to an area of lower concentration, down the concentration gradient and energetically favorable. Depending on the chemical nature of the substance, different processes may be associated with passive transport.
Diffusion
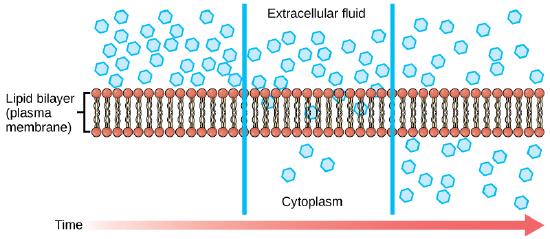
Diffusion is a passive process of transport. A single substance tends to move from an area of high concentration to an area of low concentration until the concentration is equal across a space. You are familiar with diffusion of substances through the air. For example, think about someone opening a bottle of ammonia in a room filled with people. The ammonia gas is at its highest concentration in the bottle; its lowest concentration is at the edges of the room. The ammonia vapor will diffuse, or spread away, from the bottle, and gradually, more and more people will smell the ammonia as it spreads. Materials move within the cell’s cytosol by diffusion, and certain materials move through the plasma membrane by diffusion.
Figure 1. Diffusion through a permeable membrane moves a substance from an area of high concentration (extracellular fluid, in this case) down its concentration gradient (into the cytoplasm). Each separate substance in a medium, such as the extracellular fluid, has its own concentration gradient, independent of the concentration gradients of other materials. In addition, each substance will diffuse according to that gradient. Within a system, there will be different rates of diffusion of the different substances in the medium (Attribution: Mariana Ruiz Villareal, modified)
Factors That Affect Diffusion
If unconstrained, molecules will move through and explore space randomly at a rate that depends on their size, their shape, their environment, and their thermal energy. This type of movement underlies the diffusive movement of molecules through whatever medium they are in. The absence of a concentration gradient does not mean that this movement will stop, just that there may be no net movement of the number of molecules from one area to another, a condition known as dynamic equilibrium.
Factors influencing diffusion include:
- Extent of the concentration gradient: The greater the difference in concentration, the more rapid the diffusion. The closer the distribution of the material gets to equilibrium, the slower the rate of diffusion becomes.
- Shape, size and mass of the molecules diffusing: Large and heavier molecules move more slowly; therefore, they diffuse more slowly. The reverse is typically true for smaller, lighter molecules.
- Temperature: Higher temperatures increase the energy and therefore the movement of the molecules, increasing the rate of diffusion. Lower temperatures decrease the energy of the molecules, thus decreasing the rate of diffusion.
- Solvent density: As the density of a solvent increases, the rate of diffusion decreases. The molecules slow down because they have a more difficult time getting through the denser medium. If the medium is less dense, rates of diffusion increase. Since cells primarily use diffusion to move materials within the cytoplasm, any increase in the cytoplasm’s density will decrease the rate at which materials move in the cytoplasm.
- Solubility: As discussed earlier, nonpolar or lipid-soluble materials pass through plasma membranes more easily than polar materials, allowing a faster rate of diffusion.
- Surface area and thickness of the plasma membrane: Increased surface area increases the rate of diffusion, whereas a thicker membrane reduces it.
- Distance traveled: The greater the distance that a substance must travel, the slower the rate of diffusion. This places an upper limitation on cell size. A large, spherical cell will die because nutrients or waste cannot reach or leave the center of the cell, respectively. Therefore, cells must either be small in size, as in the case of many prokaryotes, or be flattened, as with many single-celled eukaryotes.
Facilitated transport
In facilitated transport, also called facilitated diffusion, materials diffuse across the plasma membrane with the help of membrane proteins. A concentration gradient exists that allows these materials to diffuse into or out of the cell without expending cellular energy. In the case that the materials are ions or polar molecules, compounds that are repelled by the hydrophobic parts of the cell membrane, facilitated transport proteins help shield these materials from the repulsive force of the membrane, allowing them to diffuse into the cell.
Possible NB Discussion  Point
Point
Compare and contrast passive diffusion and facilitated diffusion.
Channels
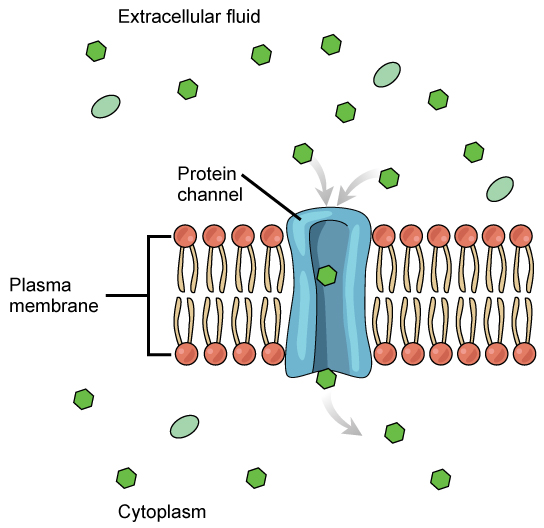
The integral proteins involved in facilitated transport are collectively referred to as transport proteins, and they function as either channels for the material or carriers. In both cases, they are transmembrane proteins. Different channel proteins have different transport properties. Some have evolved to be have very high specificity for the substance that is being transported while others transport a variety of molecules sharing some common characteristic(s). The interior "passageway" of channel proteins have evolved to provide a low energetic barrier for transport of substances across the membrane through the complementary arrangement of amino acid functional groups (of both backbone and side-chains). Passage through the channel allows polar compounds to avoid the nonpolar central layer of the plasma membrane that would otherwise slow or prevent their entry into the cell. While at any one time significant amounts of water crosses the membrane both in and out the rate of individual water molecule transport may not be fast enough to adapt to changing environmental conditions. For such cases Nature has evolved a special class of membrane proteins called aquaporins that allow water to pass through the membrane at a very high rate.
Figure 2: Facilitated transport moves substances down their concentration gradients. They may cross the plasma membrane with the aid of channel proteins. (Attribution: Mariana Ruiz Villareal, modified.)
Channel proteins are either open at all times or they are “gated.” The latter controls the opening of the channel. Various mechanisms may be involved in the gating mechanism. For instance, the attachment of a specific ion or small molecule to the channel protein may trigger opening. Changes in local membrane "stress" or changes in voltage across the membrane may also be triggers to open or close a channel.
Different organisms and tissues in multicellular species express different sets of channel proteins in their membranes depending on the environments they live in or specialized function they play in an organisms. This provides each type of cell with a unique membrane permeability profile that is evolved to complement its "needs" (note the anthropomorphism). For example, in some tissues, sodium and chloride ions pass freely through open channels, whereas in other tissues a gate must be opened to allow passage. This occurs in the kidney, where both forms of channels are found in different parts of the renal tubules. Cells involved in the transmission of electrical impulses, such as nerve and muscle cells, have gated channels for sodium, potassium, and calcium in their membranes. Opening and closing of these channels changes the relative concentrations on opposing sides of the membrane of these ions, resulting a change in electrical potential across the membrane that lead to message propagation in the case of nerve cells or in muscle contraction in the case of muscle cells.
Carrier Proteins
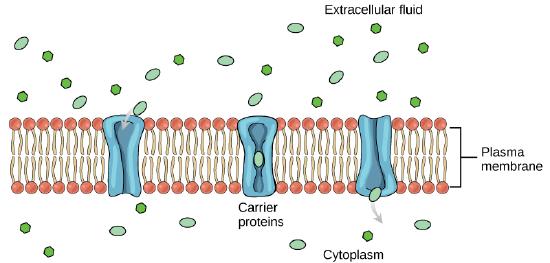
Another type of protein embedded in the plasma membrane is a carrier protein. This aptly named protein binds a substance and, in doing so, triggers a change of its own shape, moving the bound molecule from the outside of the cell to its interior; depending on the gradient, the material may move in the opposite direction. Carrier proteins are typically specific for a single substance. This selectivity adds to the overall selectivity of the plasma membrane. The molecular-scale mechanism of function for these proteins remains poorly understood.
Figure 3: Some substances are able to move down their concentration gradient across the plasma membrane with the aid of carrier proteins. Carrier proteins change shape as they move molecules across the membrane. (Attribution: Mariana Ruiz Villareal, modified.)
Carrier proteins play an important role in the function of kidneys. Glucose, water, salts, ions, and amino acids needed by the body are filtered in one part of the kidney. This filtrate, which includes glucose, is then reabsorbed in another part of the kidney with the help of carrier proteins. Because there are only a finite number of carrier proteins for glucose, if more glucose is present in the filtrate than the proteins can handle, the excess is not reabsorbed and it is excreted from the body in the urine. In a diabetic individual, this is described as “spilling glucose into the urine.” A different group of carrier proteins called glucose transport proteins, or GLUTs, are involved in transporting glucose and other hexose sugars through plasma membranes within the body.
Channel and carrier proteins transport materials at different rates. Channel proteins transport much more quickly than do carrier proteins. Channel proteins facilitate diffusion at a rate of tens of millions of molecules per second, whereas carrier proteins work at a rate of a thousand to a million molecules per second.
Note: A note of appreciation
The rates of transport just discussed are astounding. Recall that these molecular catalysts are on the scale of 10s of nanometers (10-9 meters) and that they are composed of a self-folding string of 20 amino acids and the relatively small selection of chemical functional groups that they carry.
 Active Transport
Active Transport
Active transport mechanisms require the use of the cell’s energy, usually in the form of adenosine triphosphate (ATP). If a substance must move into the cell against its concentration gradient—that is, if the concentration of the substance inside the cell is greater than its concentration in the extracellular fluid (and vice versa)—the cell must use energy to move the substance. Some active transport mechanisms move small-molecular weight materials, such as ions, through the membrane. Other mechanisms transport much larger molecules.
Moving Against a Gradient
To move substances against a concentration or electrochemical gradient, the cell must use energy. This energy is harvested from ATP generated through the cell’s metabolism. Active transport mechanisms, collectively called pumps, work against electrochemical gradients. Small substances constantly pass through plasma membranes. Active transport maintains concentrations of ions and other substances needed by living cells in the face of these passive movements. Much of a cell’s supply of metabolic energy may be spent maintaining these processes. (Most of a red blood cell’s metabolic energy is used to maintain the imbalance between exterior and interior sodium and potassium levels required by the cell.) Because active transport mechanisms depend on a cell’s metabolism for energy, they are sensitive to many metabolic poisons that interfere with the supply of ATP.
Two mechanisms exist for the transport of small-molecular weight material and small molecules. Primary active transport moves ions across a membrane and creates a difference in charge across that membrane, which is directly dependent on ATP. Secondary active transport describes the movement of material that is due to the electrochemical gradient established by primary active transport that does not directly require ATP.
Carrier Proteins for Active Transport
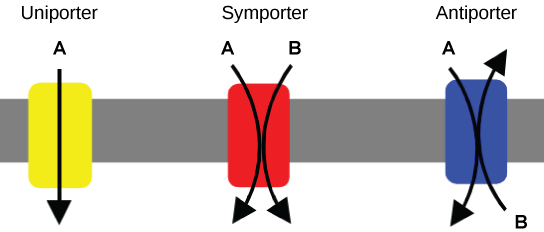
An important membrane adaption for active transport is the presence of specific carrier proteins or pumps to facilitate movement: there are three types of these proteins or transporters. A uniporter carries one specific ion or molecule. A symporter carries two different ions or molecules, both in the same direction. An antiporter also carries two different ions or molecules, but in different directions. All of these transporters can also transport small, uncharged organic molecules like glucose. These three types of carrier proteins are also found in facilitated diffusion, but they do not require ATP to work in that process. Some examples of pumps for active transport are Na+-K+ ATPase, which carries sodium and potassium ions, and H+-K+ ATPase, which carries hydrogen and potassium ions. Both of these are antiporter carrier proteins. Two other carrier proteins are Ca2+ ATPase and H+ ATPase, which carry only calcium and only hydrogen ions, respectively. Both are pumps.
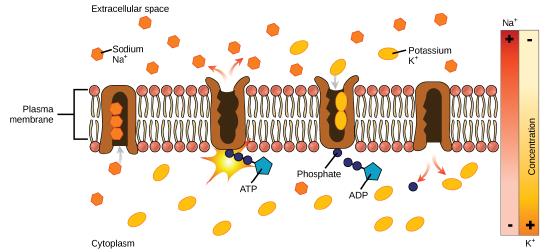
Figure 1: A uniporter carries one molecule or ion. A symporter carries two different molecules or ions, both in the same direction. An antiporter also carries two different molecules or ions, but in different directions. (credit: modification of work by “Lupask”/Wikimedia Commons)
Primary Active Transport
In primary active transport, the energy is derived directly from the breakdown of ATP. Often times, primary active transport such as that shown below which functions to transport sodium and potassium ions allows secondary active transport to occur (discussed in the section below). The second transport method is still considered active because it depends on the use of energy from the primary transport.
Figure 2: Primary active transport moves ions across a membrane, creating an electrochemical gradient (electrogenic transport). (credit: modification of work by Mariana Ruiz Villareal)
One of the most important pumps in animal cells is the sodium-potassium pump (Na+-K+ ATPase), which maintains the electrochemical gradient (and the correct concentrations of Na+ and K+) in living cells. The sodium-potassium pump moves K+ into the cell while moving Na+ out at the same time, at a ratio of three Na+ for every two K+ ions moved in. The Na+-K+ ATPase exists in two forms, depending on its orientation to the interior or exterior of the cell and its affinity for either sodium or potassium ions. The process consists of the following six steps.
- With the enzyme oriented towards the interior of the cell, the carrier has a high affinity for sodium ions. Three ions bind to the protein.
- ATP is hydrolyzed by the protein carrier and a low-energy phosphate group attaches to it.
- As a result, the carrier changes shape and re-orients itself towards the exterior of the membrane. The protein’s affinity for sodium decreases and the three sodium ions leave the carrier.
- The shape change increases the carrier’s affinity for potassium ions, and two such ions attach to the protein. Subsequently, the low-energy phosphate group detaches from the carrier.
- With the phosphate group removed and potassium ions attached, the carrier protein repositions itself towards the interior of the cell.
- The carrier protein, in its new configuration, has a decreased affinity for potassium, and the two ions are released into the cytoplasm. The protein now has a higher affinity for sodium ions, and the process starts again.
Several things have happened as a result of this process. At this point, there are more sodium ions outside of the cell than inside and more potassium ions inside than out. For every three ions of sodium that move out, two ions of potassium move in. This results in the interior being slightly more negative relative to the exterior. This difference in charge is important in creating the conditions necessary for the secondary process. The sodium-potassium pump is, therefore, an electrogenic pump (a pump that creates a charge imbalance), creating an electrical imbalance across the membrane and contributing to the membrane potential.
Link to Learning
Visit the site to see a simulation of active transport in a sodium-potassium ATPase.
Secondary Active Transport (Co-transport)
Secondary active transport brings sodium ions, and possibly other compounds, into the cell. As sodium ion concentrations build outside of the plasma membrane because of the action of the primary active transport process, an electrochemical gradient is created. If a channel protein exists and is open, the sodium ions will be pulled through the membrane. This movement is used to transport other substances that can attach themselves to the transport protein through the membrane. Many amino acids, as well as glucose, enter a cell this way. This secondary process is also used to store high-energy hydrogen ions in the mitochondria of plant and animal cells for the production of ATP. The potential energy that accumulates in the stored hydrogen ions is translated into kinetic energy as the ions surge through the channel protein ATP synthase, and that energy is used to convert ADP into ATP.
Metabolism in General Biology
Cellular metabolism represents roughly 1/3 of the General Biology curriculum. While this may seem like a lot, we cover very little of what a classic course in metabolism covers, and a minuscule fraction of the metabolism that occurs on the planet. What we cover, however, is the very important foundational knowledge. You will learn about common chemical reactions that are associated with the transformation of life's molecular building blocks and about different core modes of energy transfer that you will encounter often in biology. The energy story and the design challenge rubrics introduced earlier will become increasingly important in these next few modules and beyond.
What have we learned? How will it relate to metabolism?
- We have focused on the identification and chemical properties of common biological functional groups. As we dive into metabolism, this will help you be familiar with and sometimes even predict the chemical nature/reactivity of compounds you have never seen before.
- We have practiced recognizing and classifying molecules into four major functional groups. This will help you as we discuss how to build and break down these molecules.
- We have learned some basic thermodynamics. This gives us a common set of concepts with which to discuss whether a biochemical reaction or process is likely to occur, and if so in which direction and how fast. This will be critical as we consider some key reactions that take place in metabolism.
- We have learned and practiced the energy story rubric. This will allow us to study new biochemical reactions and to discuss them with a common, consistent language and approach which also reinforces the lessons we learned about thermodynamics.
An overview of this section
- We will introduce an important concept called reduction potential and you will be given the opportunity to use a redox tower. There is also a discussion on redox chemistry in your discussion manual. Use both resources.
- We will introduce two major players in metabolism, ATP and NADH. We expect you to recognize their structures if shown on an exam.
- We will cover the metabolic pathway glycolysis. Keep in mind that we want you to look at any reaction and tell us an energy story of that reaction. You should not try to memorize these pathways (though it will help to remember some big picture things - we will stress these). Often we will give you the pathway as a figure on the exams. Glycolysis produces 2 ATP via a process called substrate level phosphorylation, 2 NADH and 2 pyruvate compounds.
- We will use the reactions of the TCA cycle to create multiple examples of energy stories. The TCA cycle will also produce more ATP, NADH and oxidize glucose into CO2.
- We will look at an alternative pathway to that of the TCA cycle, fermentation. Here, for the first time, we will see NADH used as a reactant in a metabolic reaction.
- We will follow NADH to the end of its journey, as it donates its electrons to the electron transport chain (ETC). In this module, you will need to use a redox tower. The ETC produces a proton gradient. No ATP is directly generated in this process. However, the proton gradient is then used by the cell to run an enzyme called ATP synthase, which catalyzes the reaction ADP + Pi --> ATP. This method of ATP production (called oxidative phosphorylation) results in more ATP being produced than through substrate level phosphorylation.
- And finally, we will go through the process of photosynthesis.
REQUEST
As you go through the Redox section of the reading please use NB to also comment on parts of the reading that are hard to understand, confusing, and places that you think are well explained and clear. If you have suggested edits please let us know. Use the following emoji to tag these comments:

Reduction-Oxidation Reactions 
In General Biology, most of the reduction/oxidation reactions (redox) that we discuss occur in metabolic pathways (connected sets of biochemical reactions). Here, the cell breaks down the compounds it consumes into smaller parts and then reassembles these and other molecules into larger macromolecules. Redox reactions also play critical roles in energy transfer, either from the environment or within the cell, in all known forms of life. For these reasons, it is important to develop at least an intuitive understanding and appreciation for redox reactions in biology.
Most students of biology will also study reduction and oxidation reactions in their chemistry courses; these kinds of reactions are important well beyond biology. Regardless of the order in which students are introduced to this concept (chemistry first or biology first), most will find the topic presented in very different ways in chemistry and biology. That can be confusing.
Chemists often introduce the concepts of oxidation and reduction using the concept of oxidation states. See this link for more information: <https://chem.libretexts.org/Bookshel...ation_Numbers)>. Chemists usually ask students to apply a set of rules (see link) to determine the oxidation states of individual atoms in the molecules involved in a chemical reaction. The chemistry formalism defines oxidation as an increase in oxidation state and reduction as a decrease in oxidation state.
However, biologists don’t typically think about or teach redox reactions in this way. Why? We suspect it’s because most of the redox reactions encountered in biology involve a change in oxidation state that comes about trough a transfer of electron(s) between molecules. Biologists, therefore, typically define reduction as a gain of electrons and oxidation as a loss of electrons. We note that the biological electron-exchange view of redox reactions is entirely consistent with the more general definition associated strictly with changes in oxidation states. The electron-exchange model does not, however, explain redox reactions that do not involve a transfer of electrons, which sometimes occur in the context of a chemistry class. The biologist's view of redox chemistry has the advantage (in the context of biology) of being relatively easy to create a mental picture for. There are no lists of rules to remember or much inspection of molecular structure involved in developing at least a basic conceptual picture of the topic. We simply imagine an exchange between two parties - one molecule handing off one or more electrons to a partner who accepts them.
Since this is a biology reading for a biology class we approach redox from the “gain/loss of electrons” conceptualization. If you have already taken a chemistry class and this topic seems to be presented a little different in your biology course, remember that at its core, you are learning the same thing. Biologists just adapted what you learned in chemistry to make more intuitive sense in the context of biology. If you haven’t learned about redox, yet don’t worry. If you can understand what we are trying to do here, when you cover this concept in chemistry class you will be a few steps ahead. You will just need to work to generalize your thinking a little bit.
Let's start with some generic reactions
Transferring electrons between two compounds results in one of these compounds losing an electron and one compound gaining an electron. For example, look at the figure below. If we use the energy story rubric to look at the overall reaction, we can compare the before and after characteristics of the reactants and products. What happens to the matter (stuff) before and after the reaction? Compound A starts as neutral and becomes positively charged. Compound B starts as neutral and becomes negatively charged. Because electrons are negatively charged, we can explain this reaction with the movement of an electron from Compound A to B. That is consistent with the changes in charge. Compound A loses an electron (becoming positively charged), and we say that A has become oxidized. For biologists, oxidation is associated with the loss of electron(s). B gains the electron (becoming negatively charged), and we say that B has become reduced. Reduction is associated with the gain of electrons. We also know, since a reaction occurred (something happened), that energy must have been transferred and/or reorganized in this process and we'll consider this shortly.

Figure 1. Generic redox reaction with half-reactions Attribution: Mary O. Aina
To reiterate: When an electron(s) is lost, or a molecule is oxidized, the electron(s) must then pass to another molecule. We say that the molecule gaining the electron becomes reduced. Together these paired electron gain-loss reactions are known as an oxidation-reduction reaction (also called a redox reaction).
This idea of paired half-reactions is critical to the biological concept of redox. Electrons don’t drop out of the universe for “free” to reduce a molecule nor do they jump off a molecule into the ether. Donated electrons MUST come from a donor molecule and be transferred to some other acceptor molecule. For example, in the figure above the electron the reduces molecule B in half-reaction 2 must come from a donor - it just doesn't appear from nowhere! Likewise, the electron that leaves A in half-reaction 1 above must "land" on another molecule - it doesn't just disappear from the universe.
Therefore, oxidation and reduction reactions must ALWAYS be paired. We’ll examine this idea in more detail below when we discuss the idea of “half-reactions”.
-
A tip to help you remember: The mnemonic LEO says GER (Lose Electrons = Oxidation and Gain Electrons = Reduction) can help you remember the biological definitions of oxidation and reduction.

Figure 2. A figure for the mnemonic "LEO the lion says GER." LEO: Loss of Electrons = Oxidation. GER: Gain of Electrons = Reduction. Attribution: Kamali Sripathi
• The vocabulary of redox can be confusing: Students studying redox chemistry can often become confused by the vocabulary used to describe the reactions. Terms like oxidation/oxidant and reduction/reductant look and sound very similar but mean distinctly different things. An electron donor is also sometimes called a reductant because it is the compound that causes the reduction (gain of electrons) of another compound (the oxidant). In other words, the reductant is donating it’s electrons to the oxidant which is gaining those electrons. Conversely, the electron acceptor is called the oxidant because it is the compound that is causing the oxidation (loss of electrons) of the other compound. Again, this simply means the oxidant is gaining electrons from the reductant who is donating those electrons. Confused yet?
Yet another way to think about definitions is to remember that describing a compound as reduced/oxidized is describing the state that the compound itself is in, whereas labeling a compound as a reductant/oxidant describes how the compound can act, to either reduce or oxidize another compound. Keep in mind that the term reductant is also synonymous with reducing agent and oxidant is also synonymous with oxidizing agent. The chemists who developed this vocabulary need to be brought up on charges of "willful thickheadedness" at science trial and then be forced to explain to the rest of us why they needed to be so deliberately obtuse.
The confusing language of redox: quick summary
1. A compound can be described as “reduced” - term used to describe the compound's state.
2. A compound can be a “reductant” - term used to describe a compound's capability (it can reduce something else). The synonymous term "reducing agent" can be used to describe the same capability (the term "agent" refers to the thing that can "do something" - in this case reduce another molecule).
3. A compound can be an “oxidant” - term used to describe a compound's capability (it can oxidize something else). The synonymous term "oxidizing agent" can be used to describe the same capability (the term "agent" refers to the thing that can "do something" - in this case oxidize another molecule).
4. A compound can “become reduced” or "become oxidized"- term used to describe the transition to a new state.
Since all of these terms are used in biology, in General Biology we expect you to become familiar with this terminology. Try to learn it and use it as soon as possible - we will use the terms frequently and will not have the time to define terms each time.
Knowledge Check Quiz
Knowledge Check Quiz
The Half Reaction
Here we introduce the concept of the half reaction. We can think each half reaction as a description of what happens to one of the two molecules (i.e. the donor or the acceptor) involved in a "full" redox reaction. A "full" redox reaction requires two half reactions. In the example below, half reaction #1 depicts the molecule AH losing two electrons and a proton and in the process becoming A+. This reaction depicts the oxidation of AH. Half reaction #2 depicts the molecule B+ gaining two electrons and a proton to become BH. This reaction depicts the reduction of B+. Each of these two half reactions is conceptual and neither can happen on its own. The electrons lost in half reaction #1 MUST go somewhere, they can't just disappear. Likewise, the electrons gained in half reaction #2 must come from something. They too just can not appear out of nowhere.
One can imagine that there might be different molecules that can serve as potential acceptors (the place for the electrons to go) for the electrons lost in half reaction #1. Likewise, there might be many potential reduced molecules that can serve as the electron donors (the source of electrons) for half reaction #2. In the example below, we show what happens (the reaction) when molecule AH is the donor of electrons for molecule B+. When we put the donor and acceptor half reactions together, we get a "full" redox reaction. In the figure below we call that reaction "Reaction #1". When this happens, we say that the two half reactions coupled. NOTE: In Chemistry class, because there are sometimes more than two reactions involved in a complete redox reaction, instead of the term "coupled reaction", you might encounter the more generic terms "simultaneous reactions" or "consecutive reactions". In all cases, these terms are meant to communicate that the reactions must happen together, either at the same time or in sequence.

Figure 3. Generic redox reaction where compound AH is being oxidized by compound B+. Each half reaction represents a single species or compound to either lose or gain electrons (and a subsequent proton as shown in the figure above). In half reaction #1 AH loses a proton and 2 electrons: in the second half reaction, B+ gains 2 electrons and a proton. In this example HA is oxidized to A+ while B+ is reduced to BH.
Using this idea, we can theoretically couple and think about any two half reactions, one half reaction serving as the electron donor for the other half reaction that accepts the donated electrons. For instance, using the example above, we could consider coupling the reduction of B+ that happens in half reaction #2 with another half reaction describing the oxidation of the molecule NADH. In that case, the NADH would be the electron donor for B+. Likewise you could couple the oxidation of AH that happens in half reaction #1 with a half reaction describing the the reduction of hypothetical molecule Z+. You can mix-and-match half reactions together as you please provided one half is describing the oxidation of a compound (it's donating electrons) and the reduction of another compound (it's accepting the donated electrons).
-
A note on how we write full reactions versus half reactions: In the example above, when we write Reaction #1 as an equation, the 2 electrons - in Chemistry class, these electrons might be referred to as "intermediates" because they do not appear in the overall redox reaction - and the H+ that are explicitly described in the underlying half reactions, are not explicitly included in the text of the full reaction. In the reaction above you must infer that an exchange of electrons happens. This can be observed by trying to balance charges between each reactant and its corresponding product. Reactant AH becomes product A+. In this case, you can infer that some movement of electrons must have taken place. To balance the charges on this compound (make the sum of charges on each side of the equation equal) you need to add 2 electrons to the right side of the equation, one to account for the "+" charge on A+ and a second to go with the H+ that was also lost. The other reactant B+ is converted to BH. It must therefore gain 2 electrons to balance charges, one for B+ and a second for the additional H+ that was added. Together this information leads you to conclude that the most likely thing to have happened is that two electrons were exchanged between AH and B+.
-
This will also be the case for most redox reactions in biology. Fortunately, in most cases, either the context of the reaction, the presence of chemical groups often engaged in redox (e.g. metal ions), or the presence of commonly used electron carriers (e.g. NAD+/NADH, FAD+/FADH2, ferredoxin, etc.) will alert you that the reaction is of class "redox". You will be expected to learn to recognize some of these common molecules.
PRACTICE POST GUIDE
General Practice
3. Why: Many membrane proteins (particularly transporters) are enzymes that catalyze the movement of molecules of all types from one side of the membrane to the other. The discussion of how these proteins function tie together many of the topics we’ve built on so far - enzymes, catalysts, thermodynamics, membranes, interactions of functional groups, and now the new idea of coupling reactions. This exercise tries to help you make those connections.
How to practice: Create some drawings/models of membrane transport proteins. Blob models are fine but they should be annotated as instructed below. In your drawing include a model for a lipid bilayer clearly showing the polar and non-polar nature of the lipid components. The models of the proteins should include annotation/shading or other way of indicating the types of amino acid functional groups you might expect to see in (a) the exterior of the protein in contact with the lipid bilayer, (b) the regions of the exterior part of the protein that interact with the lipid polar groups, and (c) some attempt to illustrate the types of residues one might expect to find in the core of the protein depending on substrate being transported. Also indicate whether the protein should act as a channel (passive no need for external energy) or pump (active and requiring the coupling of an energy source).
Do this for: (1) a hypothetical protein that transports Cl- ions down their chemical gradient and (2) a transporter of glucose up the chemical gradient.
Explicitly note if each reaction being catalyzed is exergonic or endergonic and depict it in a reaction coordinate diagram. This will be the weekly drawing.
This exercise helps you practice learning objectives: MS.16 Predict the properties of the R-groups on amino acids located in different locations within transmembrane transporters.; CS.5 Discuss the advantages and trade-offs associated with simple diffusion, passive diffusion, facilitated diffusion, and active transport.; GC.42 Predict whether two reactions can be theoretically productively coupled by interpreting tables of standard Gibbs enthalpy (energy). GC.50 Describe why an enzyme is critical for coupling an exergonic reaction to an endergonic reaction by applying the concepts from the chemistry and thermodynamics lectures.
4. Why: Transport by an transmembrane protein is a reaction with a start/beginning and and end. This provides a wonderful context for you to to dissect what is happening by practicing the expert skill of telling an energy story for transport.
How to practice: For the proteins in exercise #4 practice telling an energy story for each model. Recall that the basic steps are (a) define start/beginning and end states (b) describe the matter and its organization before and after the reaction and how it’s changed (c) describe the energy and its distribution before and after and how it’s changed (recall that this can be summarized by noting something about ∆G) and (d) add relevant details of how the transformation may have been carried out (e.g. enzymes involved, etc.). Assume for the endergonic transport reaction that ATP hydrolysis is coupled as an energy source (as was depicted in class).
This exercise helps you practice learning objectives: Same as #3
5. Why: Like many topics you run into when learning biology, the concepts of transmembrane transport has a lot of vocabulary associated with it. It’s hard to make progress without learning terms and how to use them.
How to practice: Make sure to learn and associate the vocabulary with the types of transport depicted. This is from the lecture slide. These concepts are also in the reading and it is just a matter of memorizing the vocabulary with the different modes of transport. One good way might be to make some flash cards with terms on one side and definition on the other. Alternatively, cards with a depiction of the type of transport on one side and what it’s called on the other.
This exercise helps you practice learning objectives: Learning vocabulary is just good to do.
METABOLISM: REDOX
6. Why: Redox is central to metabolism and energy flow. This topic also has some of the seemingly easy but, nevertheless, most confusing vocabulary. It is a good idea to learn the vocabulary as quickly as possible and to associate them with any basic redox reaction.
How to practice: I can’t stress enough how important it will be for you to learn the redox chemistry vocabulary we outlined in class today. (Oxidized, reduced, oxidizing agent, reducing agent etc.) We will be using these terms frequently during this part of the course. Class, discussion, and the reading will be much more confusing if you do not have a good understanding of the terms. Remember, we don’t need to find the actual oxidation states of biomolecules—we just need to be able to recognize that electrons flow between compounds (An electron flow is a loss of an electron(s) from one compound and a corresponding gain of an electron(s) by another compound).
This exercise helps you practice learning objectives: GC.29 Given a redox reaction, identify the reducing agent, oxidizing agent, molecule that becomes oxidized, and the reduced species. Identify which species the electron(s) "starts" in, and to which species it “goes.”; GC.28 Identify biological redox reactions involving common electron carriers.
7. Why: The electron carrier NAD+/NADH is one of the most commonly used molecules in biochemical reactions. It pays to be able to recognize it. Unpacking its molecular structure into different components and building it back up can help to you construct a good mental picture and ability to recognize the molecule in different contexts.
How to practice: Figure 1 on the next page shows NAD+. Circle the part of the molecule that is derived from a carbohydrate. Add a square around a phosphate group. Point to a nitrogenous base. Account for the electron flow. On the curved arrows, label “reduction” and “oxidation”. Label the reduced form and oxidized form of NAD+. Next, note that H+ is involved. Try to predict, then look up, what the influence of pH might be on a redox reaction involving NAD+.
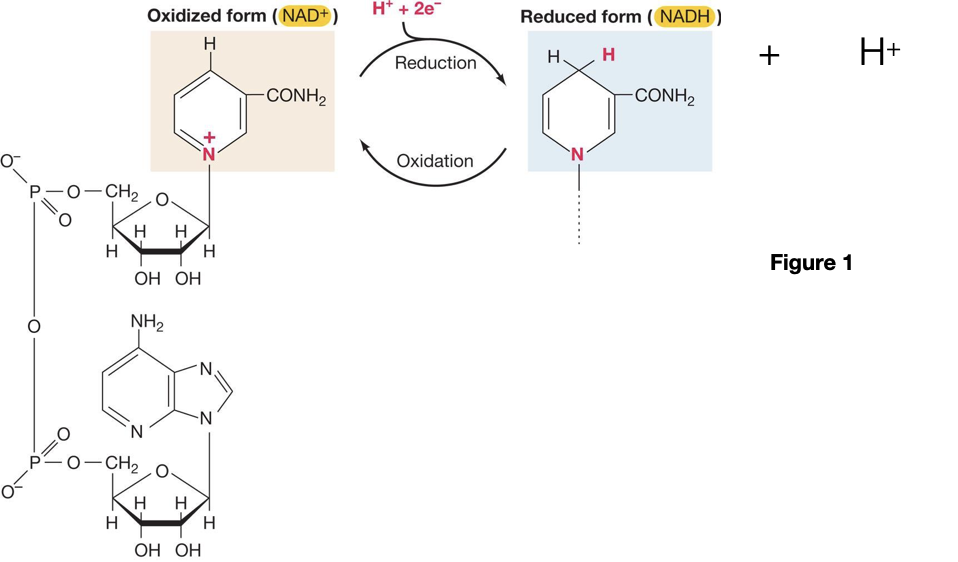
This exercise helps you practice learning objectives: ME.2 Identify NAD+ from its molecular structure and identify the functional group involved in its function as an oxidizing or reducing agent..
8. Why: Learning how to read a redox reaction is another basic skill that is necessary if you’re going to learn how this subject. The energy story rubric is a great way to walk through, step-by-step, the “parts” of a reaction.
How to practice: Understand that the energy story of a simple redox reaction is important in order to be able to answer questions about redox reactions.
Ared +Box -> Aox + Bred
- The reactants are in an initial state at the beginning of the reaction. Compound A is in its reduced form. It is the electron donor in this reaction. Compound B is in its oxidized form. It is the electron acceptor in this reaction.
- The reaction occurs. You are told that this is an exergonic reaction. Draw the reaction coordinate diagram that describes the reaction. Do you need to include activation energy in the reaction coordinate diagram? Briefly justify your answer.
- The products are in the final state of the reaction. Notice that Ared becomes Aox after donating an electron to B. Similarly, Box becomes Bred after accepting the electron from A.
- Now, look at the redox table from lecture or from your discussion manual and pick two half-reactions. Write an equation that tells the reactants and the products. Then tell the reaction’s energy story.
This exercise helps you practice learning objectives: GC.29 Given a redox reaction, identify the reducing agent, oxidizing agent, molecule that becomes oxidized, and the reduced species. Identify which species the electron(s) "starts" in, and to which species it "goes."; ME.1 Tell an energy story for a redox reaction that utilizes the electron carrier NAD+/NADH as the second substrate in the simple, generic reaction scheme: AH + NAD+ -> A + NADH.
9. Why: The concept of half reactions is ubiquitous in biology and chemistry. It’s a bit tricky to grasp, however. NAD+/NADH is a critical electron carrier. This exercise walks you through unpacking the idea of half reactions in the context of NAD+/NADH.
How to practice: Understand the importance of the following two reactions in biology:
- The half-reaction H2 + NAD+ —> NADH + H+ is a reduction of NAD+; NAD+ gains electrons.
- Another way to write this reaction is NAD+ + H+ + 2e- —> NADH + H+
- This half-reaction could be paired with an oxidation half-reaction.
- To determine the ΔE and ΔG of the pairing, you would need to know the reduction potential of both half-reactions.
- The half-reaction NADH + H+—> H2 + NAD+ is an oxidation of NADH; NADH loses electrons.
- Another way to write this reaction is NADH + H+ —> NAD+ + H+ + 2e-
- This half-reaction could be paired with a reduction half-reaction.
- To determine the ΔE and ΔG of the pairing, you would need to know the reduction potential of both half-reactions.
This exercise helps you practice learning objectives: GC.30 Write a composite chemical equation when given two redox half-reactions.; ME.1 Tell an energy story for a redox reaction that utilizes the electron carrier NAD+/NADH as the second substrate in the simple, generic reaction scheme: AH + NAD+ -> A + NADH.
10. Why: All redox reactions are associated with a change in free energy. The change in free energy can be a very important property of a given redox reaction. It will determine whether the reaction is spontaneous, it will determine whether the reaction can be coupled to another reaction productively, and so on. Here we practice learning how to recognize the relationship between ∆E of a redox reaction and its ∆G.
How to practice: Look at the formula that relates the change in free energy to the change in reduction potential: ΔG= -nF(ΔE) Fill out the table below. Is the ΔG positive, negative or zero? Is the reaction exergonic or endergonic? What happens if ΔE=0?
|
ΔE |
ΔG |
Spontaneous (exergonic) as written? |
|
>0 |
|
|
|
<0 |
|
|
|
=0 |
|
|
This exercise helps you practice learning objectives: GC.43 Qualitatively relate the difference in redox potentials with a corresponding delta of Gibbs enthalpy (energy).; GC.45 Convert between ΔG0’ and ΔE0’ for a given redox reaction using the equation ΔG0’ = -nFΔE0’.; GC.44 Define and correctly use each variable and its role in the equation: ΔG0’ = -nFΔE0’.; GC.31 Calculate the ΔE0’ for a given redox reaction using the equation ΔE0’ = E0’(oxidant) - E0’(reductant).
11. Why: This is more practice with half reactions and NAD+/NADH. It also relates the ideas of redox, half reactions and NAD+/NADH back to the concept of free energy and reaction coordinate diagrams.
How to practice: Below, draw the reaction coordinate diagram for the half-reaction H2 + NAD+ —> NADH + H+ coupled to the oxidation half-reaction A —> B. Explain the key features of this reaction and why it is important for cells. Now draw the reaction coordinate diagram for the half-reaction NADH + H+ —> H2 + NAD+ coupled to the reduction half-reaction C —> D. Explain the key features of this reaction and why it is important to cells.
This exercise helps you practice learning objectives: ME.1 Tell an energy story for a redox reaction that utilizes the electron carrier NAD+/NADH as the second substrate in the simple, generic reaction scheme: AH + NAD+ -> A + NADH.;