S2024_Bis2A_Namekawa_Posttranslational_Modifications_and_Epigenetics
- Page ID
- 132379
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Post-Translational Modifications (PTMs)
Post-Translational Modifications (PTMs) overview, Protein Activity, and Longevity
Not to be outdone by nucleic acids, proteins can also be chemically modified with the addition of groups including methyl, phosphate, acetyl, and ubiquitin groups. The addition or removal of these groups from proteins can regulate their activity or the length of time they exist in the cell. Sometimes these modifications can regulate where a protein is found in the cell—for example, in the nucleus, the cytoplasm, or attached to the plasma membrane.
Post-Translational modifications (PTMs) can occur in response to external stimuli such as stress, the lack of nutrients, heat, or ultraviolet light exposure. In addition to regulating the function of the proteins themselves, if these changes occur on specific proteins they can alter epigenetic accessibility (in the case of histone modification), transcription (transcription factors), mRNA stability (RNA binding proteins), or translation (eIF-2) thus feeding back and regulating various parts of the process of gene expression. In the case of modification to regulatory proteins, this can be an efficient way for the cell to rapidly change the levels of specific proteins in response to the environment by regulating various steps in the process.
The human genome contains approximately 20,000 to 25,000 genes. When analyzing the transcriptome, it becomes apparent that the genome becomes amplified by the wide array of splice variants that can occur during the processing of transcripts. It is estimated that the transcriptome contains roughly 100,000 transcripts. This is amplified again within the proteome that contains over 1,000,000 unique proteins. One of the main routes of proteome expansion is through PTMs of proteins. PTMs are present in both eukaryotes and prokaryotes, but it is estimated that PTMs are more common in eukaryotic cells, in which about 5% of the genome is dedicated to enzymes that carry out posttranslational modifications of proteins.
Protein PTM results from the enzymatic or nonenzymatic attachment of specific chemical groups to amino acid side chains. Such modifications occur either following protein translation or concomitant with translation. PTM influences both protein structure and physiological and cellular functions. Examples of enzymatic PTMs include phosphorylation, glycosylation, acetylation, methylation, sumoylation, and ubiquitination. Note that many of these modifications are not made in isolation. It is common for proteins to have several different types of modifications and that these modifications can differ depending on the tissue type and environmental circumstances present.
PTMs can exert their effect through a many different structural changes, including opening and closing the active site, changing the conformation and electrostatic properties of binding sites, changing the flexibility of the chain including increasing or decreasing intrinsically disordered regions, altering protein:protein interactions, etc. These effecs can also arise from the binding of small molecules.
Protein Phosphorylation
One of the most common posttranslational modifications, protein phosphorylation, is the reversible addition of a phosphoryl group from adenosine triphosphate (ATP) to amino acid side chains such as serine, threonine, and tyrosine residues. This modification causes conformational changes that either (1) affect the catalytic activity to activate or inactivate the protein and/or cause the tendency of a protein to misfold and aggregate or (2) recruit other proteins to bind; both responses result in altered protein function and cell signaling. Phosphorylated proteins have critical and well-known functions in diverse cellular processes across eukaryotes, but phosphorylation also occurs in prokaryotic cells. In humans, about one-third of proteins are estimated to be substrates for phosphorylation. Indeed, phosphorylated proteins are now identified and characterized by high-throughput phosphoproteomics studies. The reversibility of protein phosphorylation is attributed to the actions of kinases and phosphatases, which phosphorylate and dephosphorylate substrates, respectively. The temporal and spatial balance of kinase and phosphatase concentrations within a cell mediates the size of its phosphoproteome.
Protein Acetylation
The simplest form of acetylation is protein N-Acetylation. This occurs at the amino terminus amine and the ε-amino group of the lysine side chains through the action of acetylases. The acetylation of lysine side chains can be reversed through the actions of deacetylases (similar to the the combined actions of protein kinases and phosphatases). Interestingly, 80–90% of eukaryotic proteins are acetylated, yet the underlying biological significance remains unclear.
Although first described in histones, acetylation is also observed in cytoplasmic proteins. Acetylated proteins can also be modulated by the cross-talk with other posttranslational modifications, including phosphorylation, ubiquitination, and methylation. Therefore, acetylation may contribute to cell biology beyond transcriptional regulation.
Protein Glycosylation
Protein glycosylation involves the addition of a diverse set of sugar moieties to the protein core. Glycosylation has significant implications for protein folding, conformation, distribution, stability, and activity. Glycosylated proteins can have additions of simple monosaccharides. For example, many nuclear transcription factors are modified in this way. Alternatively, some proteins are modified with highly complex branched polysaccharides, such as those seen on cell surface protein receptors.
More than half of all mammalian proteins are believed to be glycosylated. However, glycoprotein functions, at both molecular and cellular levels, remain unclear. While proteins exhibit improved stability and trafficking after glycosylation in vivo, glycan structures can alter protein functions or activities. These structures often result from the activities of glycan-processing enzymes working within a cell at any given time. However, the structures are sometimes protein-specific, depending on protein trafficking properties and interactions with other cellular factors.
Protein Ubiquitination and Sumoylation
The addition of an ubiquitin group has another function - it marks that protein for degradation. Ubiquitin is a small molecule (an 8 kDa polypeptide) that acts like a flag indicating that the tagged proteins should be targeted to an organelle called the proteasome. This organelle is a large multi-protein complex that functions to cleave proteins into smaller pieces that can then be recycled. Ubiquitination (the addition of a ubiquitin tag), therefore helps to control gene expression by altering the functional lifetime of the protein product.
The addition of one ubiquitin can lead to the addition of more ubiquitins to form ubiquitin chains on the target protein. If the protein target is monoubiquinated, the activity of the target protein can be modified. For example monoubiquitination of histone proteins promotes the release the DNA enabling transcription. However, if multiple ubiquitins are added to the target protein forming a polyubiquitinated protein, the protein is tagged for degradation by the 26S proteasome.
Similarly, protein sumoylation is a reversible posttranslational modification whereby a small ubiquitin-like modifier (SUMO) protein is covalently attached to target. Like ubiquitin, SUMO is conjugated to the lysine side chains of target proteins, and it is removed by SUMO-specific isopeptidases. Sumoylation controls many aspects of nuclear function. However, recent research has started to unveil a determinant role of protein sumoylation in many extranuclear neuronal processes and potentially in a wide range of neuropathological conditions.
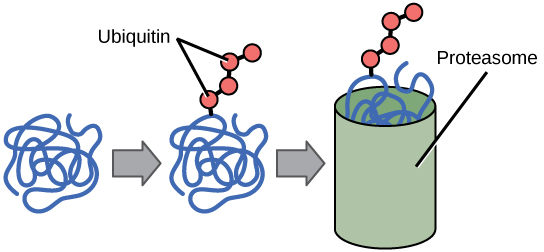
Figure 2: Proteins with ubiquitin tags are marked for degradation within the proteasome.
Histone Modifications
Histones, positively charged protein found in eukaryotic cell nuclei, pack and order the DNA into structural units called nucleosomes. How the histone proteins move depends on chemical signals found on both the histone proteins and on the DNA. These chemical signals are chemical tags added to histone proteins and the DNA that tell the histones if a chromosomal region should be "open" or "closed". The figure below depicts modifications to histone proteins and DNA. These tags are not permanent, but may be added or removed as needed. They are chemical modifications (phosphate, methyl, or acetyl groups) that attach to specific amino acids in the histone proteins or to the nucleotides of the DNA. The tags do not alter the DNA base sequence, but they do alter how tightly wound the DNA is around the histone proteins. DNA is a negatively charged molecule; therefore, changes in the histone's charge will change how tightly wound the DNA molecule will be. When unmodified, the histone proteins have a large positive charge; by adding chemical modifications like acetyl groups, the charge becomes less positive.Figure 6.6.13
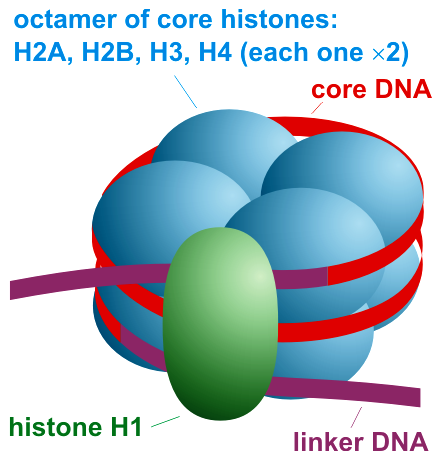
Figure 3: Nucleosome organization. https://commons.wikimedia.org/wiki/F...ganization.png. Creative Commons Attribution-Share Alike 3.0 Unported license.
The histones have multiple lysine and arginine residues that interact with the negatively charged phosphate groups of the DNA backbone. The nucleosome core is formed of two H2A-H2B dimers and an H3-H4 tetramer, forming two nearly symmetrical halves by tertiary structure (C2 symmetry; one macromolecule is the mirror image of the other). The 4 'core' histones (H2A, H2B, H3 and H4) are relatively similar in structure and are highly conserved through evolution, all featuring a 'helix turn helix turn helix' motif (a DNA-binding protein motif that recognize specific DNA sequence). They also share the feature of long 'tails' on one end of the amino acid structure - this being the location of post-translational modification, specifically N-acetylation.

Figure 4: Nucleosomes can slide along DNA. When nucleosomes are spaced closely together (top), transcription factors cannot bind and gene expression is turned off. When the nucleosomes are spaced far apart (bottom), the DNA is exposed. Transcription factors can bind, allowing gene expression to occur. Modifications to the histones and DNA affect nucleosome spacing.
Epigenetics
Epigenetics can be defined as a change in phenotype that is heritable but does not involve a change in the nucleotide sequence in DNA; that is, a change in genotype. This definition is very broad encompassing a variety of phenomena.
The word “epigenetics” has become popular in the last decade and its meaning has become confused. The term epigenetics describes any heritable change in phenotype that is not associated with a change the chromosomal DNA sequence.
Originally it meant the processes through which the genes were expressed to give the phenotype; that is, the changes in gene expression that occur during normal development of multicellular organisms. This includes the change in transcriptional state of a DNA sequence (gene) via DNA or chromatin protein reversible modifications. Thus, DNA methylation and chromatin protein methylation, phosphorylation, and acetylation have been targeted as mechanisms for “heritable” changes in cells as they grow from a single cell (zygote) and differentiate to a multicellular organism. Here, dividing cells commit to differentiate into different tissues such as muscle, neuron, and fibroblast due to the genes that they express or silence. Some genes are irreversibly silenced, through epigenetic mechanisms, in some cell types, but not in others. This doesn’t involve any change in DNA sequence.
Remember, these epigenetic effects are not permanent changes and thus cannot be selectable in an evolutionary context. However, mutations in the genes that regulate the epigenetic effect can be selected.
Epigenetic changes during cellular differentiation
For example, a change in phenotype of a single cell that is then passed on to its descendants qualifies as an epigenetic phenomenon. Thus it includes the various pathways of differentiation that are taken by cells during the embryonic development of an organism. Examples:
- X-inactivation — where one of the two X chromosomes in female mammals is inactivated in each cell early in development and that same chromosome remains inactivated in all the descendants of that cell.
- Imprinting — where whether a gene in a cell lineage is expressed or not depends on which parent contributed the gene.
The great stumbling block in converting differentiated cells into induced pluripotent stem cells (iPSCs) was to find ways of reversing the epigenetic changes in the differentiated cell (e.g., a skin cell) to unlock its full developmental potential. Stable changes in gene expression are brought about in two main ways:
- DNA methylation — where its cytosines are methylated. This usually represses the activity of that DNA.
- Histone modifications — where methyl, acetyl, and other groups are added to the histones in chromatin. Prominent examples:
- adding methyl groups to the #4 lysine in histone H3 ("H3K4me"). This is associated with active genes in that region of the chromatin.
- adding methyl groups to the #27 lysine in histone H3 ("HeK27me"). This is associated with gene silencing.
Some definitions:
- epigenetic "writers": enzymes that add chemical groups to histones or DNA.
- epigenetic "erasers": enzymes that remove these groups.
- epigenetic "readers": proteins that recognize specific epigenetic modifications of histones or DNA producing a change in gene expression, e.g., increasing (or decreasing) gene transcription.
DNA Methylation
The DNA molecule itself can also be modified. This occurs within very specific regions called CpG islands. These are stretches with a high frequency of cytosine and guanine dinucleotide DNA pairs (CG) often found in the promoter regions of genes. When this configuration exists, the cytosine member of the pair can be methylated (a methyl group is added). This modification changes how the DNA interacts with proteins, including the histone proteins that control access to the region. Highly methylated (hypermethylated) DNA regions with deacetylated histones are tightly coiled and transcriptionally inactive. DNA methylation at CpG sites can be maintained through cell divisions because there is a maintenance mechanism by copying DNA methylation patterns to the daughter strands during DNA replication; thereby, CpG methylation can serve as an epigenetic mechanism through cell divisions.
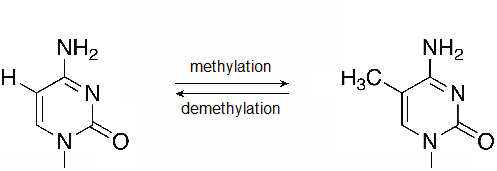
Figure 56.2.2
A methylation reaction produces 5-methylcytosine (5mC). Methyl groups may also be removed by DNA demethylases. (flickr-Beardy Git-CC:AND)
Link to external resources
Posttranslational modifications
https://www.youtube.com/watch?v=AeVDoDp3llI
Epigenetics
https://www.youtube.com/watch?v=_aAhcNjmvhc

