SS1_2023_Bis2A_Facciotti_Reading_13
- Page ID
- 110681
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Learning Objectives Associated with SS1_2023_Bis2A_Facciotti_Reading_13ME.16 Apply the concept of the “conservation of mass” to metabolism by describing the different forms mass takes as it enters and leaves the cell (e.g. input: reduced molecules like glucose, lipids, proteins, etc. & output: oxidized molecules like CO2, H2O etc.). ME.15 Apply the concept of the "conservation of energy" to central metabolism. Follow energy from "sources" of electrons with relatively low reduction potentials to "sinks" with higher redox potentials, describe the major transfers of energy and how this energy is "stored" at each stage. ————-Learning objectives above are for the full course, but particularly relevant to the discussion of metabolism and thus extend well beyond this lecture——————- MS.28 Identify ATP from its molecular structure and identify the core nucleotide functional units: nitrogenous base, ribose, and phosphates. MS.27 Identify the “high-energy” bonds in ATP. GC.71 Describe how the term "high energy" is commonly used in the context of molecular bonds and what is implied by its use. That is, answer": what makes a bond "high energy" and when is it appropriate to use the term? ME.3 Create a thermodynamic argument for how ATP hydrolysis can be coupled to drive endergonic reactions. ME.4 Explain the process of substrate level phosphorylation (SLP) and identify the SLP reactions when given a collection of reactions, such as in a pathway. ME.5 Explain the important contribution of water in determining the negative ΔG0’ of the hydrolysis of a phosphoanhydride bond in ATP. ME.7 Create an "energy story" for glycolysis. The story should list the overall reactants and products, sources of energy, energy transfers, the types of reactions involved in energy transfer, and the mediators of the transformations of matter and transfers of energy. ME.17 Explain the importance of the reaction catalyzed by glyceraldehyde-3-phosphate dehydrogenase in the harvesting of energy in glycolysis. Use this mechanism and figures to unite lessons from the course: enzymes and catalysts, energy coupling, redox, functional group chemistry, etc. ME.20 Be able to interpret figures depicting the mechanism of glyceraldehyde-3-phosphate dehydrogenase and identify key steps in the reaction including the role of a catalytic histidine and the formation of a covalent thioester linkage (including its role in energy transfer). ME.21 Create an energy story for the reaction catalyzed by glyceraldehyde-3-phosphate dehydrogenase, that discusses specifically the coupling of a redox reaction to a phosphate transfer. |
ATP
An important chemical compound is adenosine triphospate (ATP). The main cellular role of ATP is as a “short-term” energy transfer device for the cell. The hydrolysis reactions that liberate one or more of ATP's phosphates are exergonic and many, many cellular proteins have evolved to interact with ATP in ways that help facilitate the transfer of energy from hydrolysis to myriad other cellular functions. In this way, ATP is often called the “energy currency” of the cell: it has reasonably fixed values of energy to transfer to or from itself and can exchange that energy between many potential donors and acceptors. We will see many examples of ATP "at work" in the cell, so be looking for them. As you see them, try to think of them as functional examples of Nature's uses for ATP that you could expect to see in another reaction or context.
ATP structure and function
At the heart of ATP is the nucleotide called adenosine monophosphate (AMP). Like the other nucleotides, AMP is composed of a nitrogenous base (an adenine molecule) bonded to a ribose molecule and a single phosphate group. The addition of a second phosphate group to this core molecule results in the formation of adenosine diphosphate (ADP); the addition of a third phosphate group forms adenosine triphosphate (ATP).
Figure 1. ATP (adenosine triphosphate) has three phosphate groups that can be removed by hydrolysis to form ADP (adenosine diphosphate) or AMP (adenosine monophosphate).
The phosphorylation (or condensation of phosphate groups onto AMP) is an endergonic process. By contrast, the hydrolysis of one or two phosphate groups from ATP, a process called dephosphorylation, is exergonic. Why? Let's recall that the terms endergonic and exergonic refer to the sign on the difference in standard free energy of a reaction between the products and reactants, ΔG°'. Here we are explicitly assigning a direction to the reaction, either toward phosphorylation or dephosphorylation of the nucleotide. In the phosphorylation reaction, the reactants are the nucleotide and an inorganic phosphate while the products are a phosphorylated nucleotide and WATER. In the dephosphorylation/hydrolysis reaction, the reactants are the phosphorylated nucleotide and WATER while the products are inorganic phosphate and the nucleotide minus one phosphate.
Since Gibbs free energy is a state function, it doesn't matter how the reaction happens; you just consider the beginning and ending states. As an example, let's examine the hydrolysis of ATP. The reactants ATP and water are characterized by their atomic makeup and the kinds of bonds between the constituent atoms. We can associate some free energy with each of the bonds and their possible configurations—likewise for the products. If we examine the reaction from the standpoint of the products and reactants and ask "how can we recombine atoms and bonds in the reactants to get the products?," we find that a phosphoanhydride bond between an oxygen and a phosphorus must be broken in the ATP, a bond between an oxygen and hydrogen must be broken in the water, a bond must be made between the OH (that came from the splitting of water) and the phosphorus (from the freed PO3-2), and a bond must be formed between the H (derived from the splitting of water) and the terminal oxygen on the phosphorylated nucleotide. It is the sum of energies associated with all of those bond rearrangements (including those directly associated with water) that makes this reaction exergonic. We could make a similar analysis with the reverse reaction.
Is there something special about the specific bonds involved in these molecules? Much is made in various texts about the types of bonds between the phosphates of ATP. Certainly, the properties of the bonds in ATP help define the molecule's free energy and reactivity. However, while it is appropriate to apply concepts like charge density and availability of resonance structures to this discussion, trotting these terms out as an "explanation" without a thorough understanding of how these factors influence the free energy of the reactants is a special kind of hand-waving that we shouldn't engage in. Most BIS2A students have not had any college chemistry and those who have are not likely to have discussed those terms in any meaningful way. So, explaining the process using the ideas above only gives a false sense of understanding, assigns some mystical quality to ATP and its "special" bonds that don't exist, and distracts from the real point: the hydrolysis reaction is exergonic because of the properties of ATP and ALSO because of the chemical properties of water and those of the reaction products. For this class, it is sufficient to know that dedicated physical chemists are still studying the process of ATP hydrolysis in solution and in the context of proteins and that they are still trying to account for the key enthalpic and entropic components of the component free energies. We'll just need to accept a certain degree of mechanistic chemical ignorance and be content with a description of gross thermodynamic properties. The latter is perfectly sufficient to have deep discussions about the relevant biology.
"High-Energy" bonds
What about the term "high-energy bonds" that we so often hear associated with ATP? If there is nothing "special" about the bonds in ATP, why do we always hear the term "high-energy bonds" associated with the molecule? The answer is deceptively simple. In biology the term "high-energy bond" is used to describe an exergonic reaction involving the hydrolysis of the bond in question that results in a "large," negative change in free energy. Remember that this change in free energy has not only to do with the bond in question but rather the sum of all bond rearrangements in the reaction. What constitutes a large change? It is a rather arbitrary assignment usually associated with an amount of energy associated with the types of anabolic reactions we typically observe in biology. If there is something special about the bonds in ATP, it is not uniquely tied to the free energy of hydrolysis, as there are plenty of other bonds whose hydrolysis results in greater negative differences in free energy.
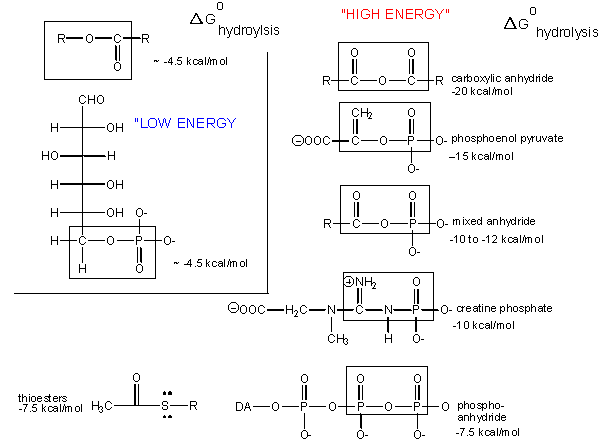
Figure 2. The standard free energy of hydrolysis of different types of bonds can be compared to that of the hydrolysis of ATP. Source: http://bio.libretexts.org/Core/Biochemistry/Oxidation_and_Phosphorylation/ATP_and_Oxidative_Phosphorylation/Properties_of_ATP

Table 1. Table of common cellular phosphorylated molecules and their respective standard free energies of hydrolysis.
External link discussing the energetics of coupling ATP hydrolysis to other reactions
Possible NB Discussion  Point
Point
You have just now read about two important molecules: NADH/NAD+ and ATP. In what biological contexts/process do you expect to see NADH/NAD+? What about ATP? Can you state what you know so far about the relationship between NADH/NAD+ and ATP? Take a moment to identify any gaps in comprehension you might have -- what questions are you left with after reading the text? Help your peers out with their questions/discussions to reinforce your own knowledge!
The cycling of ATP pools
Estimates for the number of ATP molecules in a typical human cell range from ~3x107 (~5x10-17 moles ATP/cell) in a white blood cell to 5x109 (~9x10-15 moles ATP/cell) in an active cancer cell. While these numbers might seem large, and already amazing, consider that it is estimated that this pool of ATP turns over (becomes ADP and then back to ATP) 1.5 x per minute. Extending this analysis yields the estimate that this daily turnover amounts to roughly the equivalent of one body weight of ATP getting turned over per day. That is, if no turnover/recycling of ATP happened, it would take one body weight worth of ATP for the human body to function, hence our previous characterization of ATP as a "short-term" energy transfer device for the cell.
While the pool of ATP/ADP may be recycled, some of the energy that is transferred in the many conversions between ATP, ADP, and other biomolecules is also transferred to the environment. In order to maintain cellular energy pools, energy must transfer in from the environment as well. Where does this energy come from? The answer depends a lot on where energy is available and what mechanisms Nature has evolved to transfer energy from the environment to molecular carriers like ATP. In nearly all cases, however, the mechanism of transfer has evolved to include some form of redox chemistry.
In this and the sections that follow we are concerned with learning some critical examples of energy transfer from the environment, key types of chemistry and biological reactions involved in this process, and key biological reactions and cellular components associated with energy flow between different parts of the living system. We focus first on reactions involved in the (re)generation of ATP in the cell (not those involved in the creation of the nucleotide per se but rather those associated with the transfer of phosphates onto AMP and ADP).
Video link
For another perspective - including places you'll see ATP in Bis2a, take a look at this video (10 minutes) by clicking here.
How do cells generate ATP?
A variety of mechanisms have emerged over the 3.25 billion years of evolution to create ATP from ADP and AMP. The majority of these mechanisms are modifications on two themes: direct synthesis of ATP or indirect synthesis of ATP with two basic mechanisms known respectively as substrate level phosphorylation (SLP) and oxidative phosphorylation. Both mechanisms rely on biochemical reactions that transfer energy from some energy source to ADP or AMP to synthesize ATP. These topics are substantive, so they will be discussed in detail in the next few modules.
Glycolysis: An overview
Organisms, whether unicellular or multicellular, need to find ways of getting at least two key things from their environment: (1) matter or raw materials for maintaining a cell and building new cells and (2) energy to help with the work of staying alive and reproducing. Energy and the raw materials may come from different places. For instance, organisms that primarily harvest energy from sunlight will get raw materials for building biomolecules from sources like CO2. By contract, some organisms rely on red/ox reactions with small molecules and/or reduced metals for energy and get their raw materials for building biomolecules from compounds unconnected to the energy source. Meanwhile, some organisms (including ourselves), have evolved to get energy AND the raw materials for building and cellular maintenance from sometimes associated sources.
Glycolysis is the first metabolic pathway discussed in BIS2A; a metabolic pathway is a series of linked biochemical reactions. Because of its ubiquity in biology, we hypothesize that glycolysis was probably one of the earliest metabolic pathways to evolve (more on this later). Glycolysis is a ten-step metabolic pathway that is centered on the processing of glucose for both energy extraction from chemical fuel and for the processing of the carbons in glucose into various other biomolecules (some of which are key precursors of many much more complicated biomolecules). We will therefore examine our study of glycolysis using the precepts outlined in the energy challenge rubric that ask us to formally consider what happens to BOTH matter and energy in this multi-step process.
The energy story and design challenge of glycolysis
Our investigation of glycolysis is a good opportunity to examine a biological process using both the energy story and the design challenge rubrics and perspectives.
The design challenge rubric will try to get you to think actively, and broadly and specifically, about why we are studying this pathway—what is so important about it? What "problems" does the evolution of a glycolytic pathway allow life to solve or overcome? We will also want to think about alternate ways to solve the same problems and why they may or may not have evolved. Later, we will examine a hypothesis for how this pathway—and other linked pathways—may have evolved, and thinking about alternative strategies for satisfying various constraints will come in handy then.
We ask you to think about glycolysis through the lens of an energy story in which you examine the 10-step process as a set of matter and energy inputs and outputs, a process with a beginning and an end. By taking this approach you will learn not only about glycolysis, but also some skills required to read and interpret other biochemical pathways.
So what is glycolysis? Let's find out.

Figure 1. The ten biochemical reactions of glycolysis are shown. Enzymes are labeled in blue. The structure of each sugar-derived compound is depicted as a molecular model; other reactants and products may be abbreviated (e.g., ATP, NAD+, etc.). The box surrounding the reaction catalyzed by glyceraldehyde 3-phosphate dehydrogenase indicates that this reaction is of special interest in the course. Attribution: Marc T. Facciotti (original work)
Table 1. This table shows glycolytic enzymes and measurements of the energy at standard state (ΔG°'/(kJ/mol)) compared with measurements taken from a living cell (ΔG/(kJ/mol)). Under conditions of constant temperature and pressure, (ΔG°'/(kJ/mol)), reactions will occur in the direction that leads to a decrease in the value of the Gibbs free energy. Cellular measurements of ΔG can be dramatically different from ΔG°' measurements because of cellular conditions, such as concentrations of relevant metabolites, etc. There are three large, negative ΔG drops in the cell in the process of glycolysis. We consider these reactions irreversible and are often subject to regulation
| Enzyme | Step | ΔG/(kJ/mol) | ΔG°'/(kJ/mol) |
|---|---|---|---|
| Hexokinase | 1 | -34 | -16.7 |
| Phosphoglucose isomerase | 2 | -2.9 | 1.67 |
| Phosphofructokinase | 3 | -19 | -14.2 |
| Fructose-bisphosphate aldolase | 4 | -0.23 | 23.9 |
| Triose phosphate isomerase | 5 | 2.4 | 7.56 |
| Glyceraldehyde 3-phosphate dehydrogenase | 6 | -1.29 | 6.30 |
| Phosphoglycerate kinase | 7 | 0.09 | -18.9 |
| Phosphoglycerate mutase | 8 | 0.83 | 4.4 |
| Enolase | 9 | 1.1 | 1.8 |
| Pyruvate kinase | 10 | -23.0 | -31.7 |
Overall, the glycolytic pathway comprises 10 enzyme-catalyzed steps. The primary input into this pathway is a single molecule of glucose, though we discover that other molecules may enter this pathway at various steps. We will focus our attention on (1) consequences of the overall process, (2) several key reactions that highlight important types of biochemistry and biochemical principles we will want to carry forward to other contexts, and (3) alternative fates of the intermediates and products of this pathway.
Note for reference that glycolysis is an anaerobic process. There is no requirement for molecular oxygen in glycolysis - oxygen gas is not a reactant in any of the chemical reactions in glycolysis. Glycolysis occurs in the cytosol or cytoplasm of cells. For a short (three-minute) overview YouTube video of glycolysis, click here.
First half of glycolysis: energy investment phase
We typically refer the first few steps of glycolysis as an "energy investment phase" of the pathway. This, however, doesn't make much intuitive sense (in the framework of a design challenge; it's not clear what problem this energy investment solves) if one only looks at glycolysis as an "energy-producing" pathway and until these steps of glycolysis are put into a broader metabolic context. We'll try to build that story as we go, so for now just recall that we mentioned that some first steps are often associated with energy investment and ideas like "trapping" and "commitment" that are noted in the figure below.
Step 1 of glycolysis:
The first step in glycolysis, shown below in Figure 2, is glucose being catalyzed by hexokinase, an enzyme with broad specificity that catalyzes the phosphorylation of six-carbon sugars. Hexokinase catalyzes the phosphorylation of glucose, where glucose and ATP are substrates for the reaction, producing a molecule called glucose 6-phosphate and ADP as products.
Figure 2. The first half of glycolysis is called the energy investment phase. In this phase, the cell spends two ATPs into the reactions. Attribution: Marc T. Facciotti (original work)
Note:
The paragraph above states that the enzyme hexokinase has "broad specificity." This means that it can catalyze reactions with different sugars, not just glucose. From a molecular perspective, can you explain why this might be the case? Does this challenge your conception of enzyme specificity? If you Google the term "enzyme promiscuity" (don't worry; it's safe for work), you will hopefully gain a broader appreciation for enzyme selectivity and activity.
The conversion of glucose to the negatively charged glucose 6-phosphate significantly reduces the likelihood that the phosphorylated glucose leaves the cell by diffusion across the hydrophobic interior of the plasma membrane. It also "marks" the glucose in a way that tags it for several possible fates (see Figure 3).

Figure 3. Note that this figure shows that glucose 6-phosphate can, depending on cellular conditions, be directed to multiple fates. While it is a component of the glycolytic pathway, it is not only involved in glycolysis but also in the storage of energy as glycogen (colored in cyan) and in the building of various other molecules like nucleotides (colored in red). Source: Marc T. Facciotti (original work)
As Figure 3 shows, glycolysis is but one fate for glucose 6-phosphate (G6P). Depending on cellular conditions, G6P may be diverted to the biosynthesis of glycogen (for energy storage), or it may be diverted into the pentose phosphate pathway for the biosynthesis of various biomolecules, including nucleotides. This means that G6P, while involved in the glycolytic pathway, is not solely tagged for oxidation at this phase. Perhaps showing the broader context that this molecule is involved in (in addition to the rationale that tagging glucose with a phosphate decreases the likelihood that it will leave the cell) helps to explain the seemingly contradictory (if you only consider glycolysis as an "energy-producing" process) reason for transferring energy from ATP onto glucose if it is only to be oxidized later—that is, glucose is not only used by the cell for harvesting energy and several other metabolic pathways depend on the transfer of the phosphate group.
Step 2 of glycolysis:
In the second step of glycolysis, an isomerase catalyzes the conversion of glucose 6-phosphate into one of its isomers, fructose 6-phosphate. An isomerase is an enzyme that catalyzes the conversion of a molecule into one of its isomers.
Step 3 of glycolysis:
The third step of glycolysis is the phosphorylation of fructose 6-phosphate, catalyzed by the enzyme phosphofructokinase. A second ATP molecule donates a phosphate to fructose 6-phosphate, producing fructose 1,6-bisphosphate and ADP as products. In this pathway, phosphofructokinase is a rate-limiting enzyme, and its activity is tightly regulated. It is allosterically activated by AMP when the concentration of AMP is high and when it is moderately allosterically inhibited by ATP at the same site. Citrate, a compound we'll discuss soon, also acts as a negative allosteric regulator of this enzyme. In this way, phosphofructokinase monitors or senses molecular indicators of the energy status of the cells and can in response act as a switch that turns on or off the flow of the substrate through the rest of the metabolic pathway depending on whether there is “sufficient” ATP in the system. The conversion of fructose 6-phosphate into fructose 1,6-bisphosphate is sometimes referred to as a commitment step by the cell to the oxidation of the molecule in the rest of the glycolytic pathway by creating a substrate for and helping to energetically drive the next highly endergonic (under standard conditions) step of the pathway.
Step 4 of glycolysis:
In the fourth step in glycolysis, an enzyme, fructose-bisphosphate aldolase, cleaves 1,6-bisphosphate into two three-carbon isomers: dihydroxyacetone phosphate and glyceraldehyde 3-phosphate.
Second half: energy payoff phase
If viewed in the absence of other metabolic pathways, glycolysis has so far cost the cell two ATP molecules and produced two small, three-carbon sugar molecules: dihydroxyacetone phosphate (DAP) and glyceraldehyde 3-phosphate (G3P). When viewed in a broader context, this investment of energy to produce a variety of molecules that can be used in a variety of other pathways doesn't seem like such a bad investment.
Both DAP and G3P can proceed through the second half of glycolysis. We now examine these reactions.
Figure 4. The second half of glycolysis is called the energy payoff phase. In this phase, the cell gains two ATP and two NADH compounds. At the end of this phase, glucose has become partially oxidized to form pyruvate. Attribution: Marc T. Facciotti (original work).
Step 5 of glycolysis:
In the fifth step of glycolysis, an isomerase transforms the dihydroxyacetone phosphate into its isomer, glyceraldehyde 3-phosphate. The six-carbon glucose has therefore now been converted into two phosphorylated three-carbon molecules of G3P.
Step 6 of glycolysis:
The sixth step is key and one from which we can now leverage our understanding of the several chemical reactions that we've studied so far. If you're energy focused, this is finally a step of glycolysis where some reduced sugar becomes oxidized. The reaction is catalyzed by the enzyme glyceraldehyde 3-phosphate dehydrogenase. This enzyme catalyzes a multi-step reaction between three substrates—glyceraldehyde 3-phosphate, the cofactor NAD+, and inorganic phosphate (Pi)—and produces three products: 1,3-bisphosphoglycerate, NADH, and H+. One can think of this reaction as two reactions: (1) an oxidation/reduction reaction and (2) a condensation reaction in which an inorganic phosphate is transferred onto a molecule. Here, the red/ox reaction, a transfer of electrons off G3P and onto NAD+, is exergonic, and the phosphate transfer is endergonic. The net standard free energy change hovers around zero—more on this later. The enzyme here acts as a molecular coupling agent to couple the energetics of the exergonic reaction to that of the endergonic reaction, thus driving both forward. This processes happens through a multi-step mechanism in the enzyme's active site and involves the chemical activity of a variety of functional groups.
It is important to note that this reaction depends upon the availability of the oxidized form of the electron carrier, NAD+. If we consider that there is a limiting pool of NAD+, we can then conclude that the reduced form of the carrier (NADH) must continuously oxidize back into NAD+ to keep this step going. If NAD+ is not available, the second half of glycolysis slows down or stops.
Possible NB Discussion  Point
Point
Can you write an energy story for Step 6 of glycolysis (the reaction catalyzed by glyceraldehyde 3-phosphate dehydrogenase)? When you discuss energy, just describe whether steps are exergonic or endergonic. As a group, try to build up an increasingly "expert" version that is complete, brief, and uses appropriate vocabulary. Amend one another's texts in a polite and constructive way.
Step 7 of glycolysis:
In the seventh step of glycolysis, catalyzed by phosphoglycerate kinase (an enzyme named for the reverse reaction), 1,3-bisphosphoglycerate transfers a phosphate to ADP, forming one molecule of ATP and a molecule of 3-phosphoglycerate. This reaction is exergonic and is also an example of substrate-level phosphorylation.
Step 8 of glycolysis:
In the eighth step, the remaining phosphate group in 3-phosphoglycerate moves from the third carbon to the second carbon, producing 2-phosphoglycerate (an isomer of 3-phosphoglycerate). The enzyme catalyzing this step is a mutase (isomerase).
Step 9 of glycolysis:
Enolase catalyzes the ninth step. This enzyme causes 2-phosphoglycerate to lose water from its structure; this is a dehydration reaction, resulting in the formation of a double bond that increases the potential energy in the remaining phosphate bond and produces phosphoenolpyruvate (PEP).
Step 10 of glycolysis:
The last step in glycolysis is catalyzed by the enzyme pyruvate kinase (the enzyme in this case is named for the reverse reaction of pyruvate’s conversion into PEP) and results in the production of a second ATP molecule by substrate-level phosphorylation and the compound pyruvic acid (or its salt form, pyruvate). Many enzymes in enzymatic pathways are named for the reverse reactions, since the enzyme can catalyze both forward and reverse reactions (these may have been described initially by the reverse reaction that takes place in vitro, under non-physiological conditions).
Outcomes of glycolysis
Here are a couple of things to consider:
One of the clear outcomes of glycolysis is the biosynthesis of compounds that can enter into a variety of metabolic pathways. Likewise, compounds coming from other metabolic pathways can feed into glycolysis at various points. So, this pathway can be part of a central exchange for carbon flux within the cell.
If glycolysis runs long enough, the constant oxidation of glucose with NAD+ can leave the cell with a problem: how to regenerate NAD+ from the two molecules of NADH produced. If the cell does not regenerate NAD+, nearly all the cell's NAD+ will transform into NADH. So how do cells regenerate NAD+?
Pyruvate is not completely oxidized; There is still some energy to extract. How might this happen? Also, what should the cell do with all of that NADH? Is there any energy there to extract?
Possible NB Discussion  Point
Point
To some, that glycolysis is such a complex, multi-step pathway may seem counter-intuitive: “Why wouldn’t evolution lead to a *simpler* way to extract energy from food since energy is an important requirement for life?” Explain the necessity/advantage of having glucose get broken down in many steps.
Substrate-level phosphorylation (SLP)
The simplest route to synthesize ATP is substrate-level phosphorylation. ATP molecules are generated (that is, regenerated from ADP) because of a chemical reaction that occurs in catabolic pathways. A phosphate group is removed from an intermediate reactant in the pathway, and the free energy of the reaction is used to add the third phosphate to an available ADP molecule, producing ATP. This very direct method of phosphorylation is called substrate-level phosphorylation (SLP). We can find SLP in a variety of catabolic reactions, most notably in two specific reactions in glycolysis (which we will discuss specifically later). What the reaction requires is a high-energy intermediate compound whose free energy of oxidation can drive the synthesis of ATP.
Figure 5. Here is one example of substrate-level phosphorylation occurring in glycolysis. There is a direct transfer of a phosphate group from the carbon compound onto ADP to form ATP. Attribution: Marc T. Facciotti (own work)
In this reaction, the reactants are a phosphorylated carbon compound called G3P (from step 6 of glycolysis) and an ADP molecule, and the products are 1,3-BPG and ATP. The transfer of the phosphate from G3P to ADP to form ATP in the active site of the enzyme is substrate-level phosphorylation. This occurs twice in glycolysis and once in the TCA cycle (for a subsequent reading).
PRACTICE POST GUIDE
General Practice
3. Why: I am repeating this exercise from the previous lecture - it’s that important. Redox is central to metabolism and energy flow. This topic also has some of the seemingly easy but, nevertheless, most confusing vocabulary. It is a good idea to learn the vocabulary as quickly as possible and to associate them with any basic redox reaction.
How to practice: I can’t stress enough - again - how important it will be for you to learn the vocabulary associated with redox chemistry (oxidized, reduced, oxidizing agent, reducing agent etc.). We will be using these terms frequently during this part of the course. Class, discussion, and the reading will be much more confusing if you do not have a good understanding of the terms. Remember, we don’t need to do any “fancy” chemistry and find the actual oxidation states of biomolecules—we just need to be able to recognize that there is a flow of electrons (a loss of an electron(s) from one compound and a corresponding gain of an electron(s) by another compound) between compounds.
This exercise helps you practice learning objectives: GC.29 Given a redox reaction, identify the reducing agent, oxidizing agent, molecule that becomes oxidized, and the reduced species. Identify which species the electron(s) "starts" in, and to which species it "goes."
4. Why: ATP is a very important molecule for the short-term transfer of energy, energy coupling, and transfer of phosphate functional groups. It’s also a core building block of RNA and in a deoxy-form core building block of RNA. It’s an important molecule to get to know better. Here we start by asking you to use the global textbook - the internet - to find reactions involving ATP. Google or your favorite search engine is good for that. This website <http://biochemical-pathways.com/#/map/1> allows you to browse biochemical maps - use that to find reactions involving ATP.
How to practice: Look up some metabolic pathways on the internet, in your textbook, and/or on the metabolic chart located in the learning center. See how many different reactions involve ATP/ADP? What can you say about these coupled reactions? Can you dissect the reactions you find on the metabolic charts and tell their energy stories? Do this for a few.
This exercise helps you practice learning objectives: ME.3 Create a thermodynamic argument for how ATP hydrolysis can be coupled to drive endergonic reactions.; ME.4 Explain the process of substrate level phosphorylation (SLP) and identify the SLP reactions when given a collection of reactions, such as in a pathway.
5. Why: More practice examining ATP and its role in energy transfer. Don’t need to say more.
How to practice: The diagram below shows a number of important features about ATP/ADP. Rewrite this diagram for yourself - in your notes. Take special care to deconstruct ATP into its three core parts and to identify the so-called “high-energy” bonds. Now think about the hydrolysis reaction depicted specifically. The water is not drawn explicitly in the figure - try to find where it shows up. You’ll do more in part c.
-
- The reaction ATP + 2H2O —> ADP+Pi + H3O+ is exergonic. What are some of the other important features of this reaction?
- The reaction ADP+Pi + H3O+ —> ATP + 2 H2O is endergonic. What are some of the other important features of this reaction?
- Notice that both H2O and H3O+ are not shown in the drawing. Can you draw them in?
This exercise helps you practice learning objectives: MS.28 Identify ATP from its molecular structure and identify the core nucleotide functional units: nitrogenous base, ribose, and phosphates.; MS.27 Identify the “high-energy” bonds in ATP.; ME.3 Create a thermodynamic argument for how ATP hydrolysis can be coupled to drive endergonic reactions.; ME.5 Explain the important contribution of water in determining the negative ΔG0’ of the hydrolysis of a phosphoanhydride bond in ATP.; GC.71 Describe how the term "high energy" is commonly used in the context of molecular bonds and what is implied by its use. That is, answer": what makes a bond "high energy" and when is it appropriate to use the term?

6. Why: This is extending the practice from exercise 5 but now linking back to previous skills involving reaction coordinate diagrams and thermodynamics.
How to practice: Draw the reaction coordinate diagrams for the following reactions:
-
- ATP + 2H2O —> ADP+Pi + H3O+. Explain the key features of this reaction and why it is important to cells.
- b. ADP+Pi + H3O+ —> ATP + 2H2O. Explain the key features of this reaction and why this reaction is important to cells.
This exercise helps you practice learning objectives: ME.5 Explain the important contribution of water in determining the negative ΔG0’ of the hydrolysis of a phosphoanhydride bond in ATP.
7. Why: Redox is central to the story of metabolism. In glycolysis we meet our first key redox reaction responsible for harvesting energy from the food we (and other organisms) eat. You’ve practiced recognizing redox reactions and using redox tables - here are a few reactions that we’re going to look at over the next couple of lectures to make sure we’re continuing to practice this skill.
How to practice: Based on the redox table at right, predict whether the following transfers listed below are exergonic or endergonic. Then, write out the reaction and identify the reactants and the products. Reaction (a) involves NAD+/NADH. Reactions (b) and (c) involve the small protein ferredoxin that is also involved in many electron transfers. Learn to associate this protein with this kind of chemistry.
- Transfer of electrons from acetaldehyde to NAD+.
- Transfer of electrons from succinate to ferredoxin
- Transfer of electrons from ferredoxin to pyruvate

This exercise helps you practice learning objectives: GC.31 Calculate the ΔE0’ for a given redox reaction using the equation ΔE0’ = E0’(oxidant) - E0’(reductant).; GC.29 Given a redox reaction, identify the reducing agent, oxidizing agent, molecule that becomes oxidized, and the reduced species. Identify which species the electron(s) "starts" in, and to which species it "goes."; GC.45 Convert between ΔG0’ and ΔE0’ for a given redox reaction using the equation ΔG0’ = -nFΔE0’.; GC.43 Qualitatively relate the difference in redox potentials with a corresponding delta of Gibbs enthalpy (energy).; GC.26 Predict whether a directional transfer of electrons between two chemical species is endergonic or exergonic by applying the concept of redox potential to provided data.
8. Why: The direct transfer of phosphate groups from small molecules to ADP to make ATP is known as substrate-level phosphorylation. This is one of the main mechanisms for ATP synthesis that you need to learn how to spot.
How to practice: Examine the glycolytic pathway. Identify all instances of substrate-level phosphorylation and create an energy story for those reactions.
This exercise helps you practice learning objectives: ME.4 Explain the process of substrate level phosphorylation (SLP) and identify the SLP reactions when given a collection of reactions, such as in a pathway.
9. Why: Glycolysis is a universal metabolic pathway of carbon flow and energy transfer. Here we want to start to familiarize ourselves with this set of reactions and some of its core properties. This question ask you to think about different parts of the pathway and how they connect.
How to practice: In steps 6 through 10 of glycolysis, the conversion of one mole of glyceraldehyde-3-phosphate to pyruvate yields two moles of ATP. However, the oxidation of glucose to pyruvate produces a total of four moles of ATP. Where do the remaining two moles of ATP come from?
This exercise helps you practice learning objectives: ME.7 Create an "energy story" for glycolysis. The story should list the overall reactants and products, sources of energy, energy transfers, the types of reactions involved in energy transfer, and the mediators of the transformations of matter and transfers of energy.; ME.16 Apply the concept of the “conservation of mass” to metabolism by describing the different forms mass takes as it enters and leaves the cell (e.g. input: reduced molecules like glucose, lipids, proteins, etc. & output: oxidized molecules like CO2, H2O etc.).
10. Why: It is common to discuss glycolysis as a story about energy harvesting. Of course, that’s true. However, I find that the usual stories of glycolysis spend way too much time distracting students with diversions of how many ATP are invested and how many are created and how/where to count them. These treatments, in my view, are mostly a waste of time because they don’t actually teach you anything about how energy is harvested in glycolysis - they’re all mostly bean-counting exercises. More critical, in my view, is to start understanding where energy is actually extracted and how it is extracted. That happens in the reaction in which glyceraldehyde-3-phosphate is oxidized. Let’s get to learn about it.
How to practice: You’ve seen this reaction in the context of glycolysis. Try to confidently explain this reaction to your classmate at various levels (big picture to atomic level, to energetics, water, etc.) by drawing on all the topics we’ve talked about, and use your explanation to see the connections between topics in the class. If you can do this confidently, you are likely doing well. Doing something like this may be easier at the end of the week but trying this will give you a feeling for where you stand.

This exercise helps you practice learning objectives: ME.15 Apply the concept of the "conservation of energy" to central metabolism. Follow energy from "sources" of electrons with relatively low reduction potentials to "sinks" with higher redox potentials, describe the major transfers of energy and how this energy is "stored" at each stage.; ME.17 Explain the importance of the reaction catalyzed by glyceraldehyde-3-phosphate dehydrogenase in the harvesting of energy in glycolysis. Use this mechanism and figures to unite lessons from the course: enzymes and catalysts, energy coupling, redox, functional group chemistry, etc.; ME.21 Create an energy story for the reaction catalyzed by glyceraldehyde-3-phosphate dehydrogenase, that discusses specifically the coupling of a redox reaction to a phosphate transfer..
11. Why: More practice on the reaction above. Now, however, we want to link back to older learning objectives and skills in the course - in particular here we want to explicitly link the story above to our ideas of what enzymes look like, what they’re made of, and how they function.
How to practice: First, get out your sketchbook. Draw a blob version of an enzyme and label it “glyceraldehyde 3-phosphate dehydrogenase”. Now draw/write a representation of the substrates of the reaction and use an arrow to indicate that they go into the active site of the blob enzyme. Now draw an arrow coming out of the blob and place the products of the reaction at the end of the arrow. Look carefully. Did you get ALL of the reactants and products? It’s critical you understand that one enzyme is handling all of this chemistry.
This exercise helps you practice learning objectives: MS.17 Draw a rough sketch of an enzyme including its active site and other sites in the enzyme that might impact its function, such as an inhibitor binding site.; other learning objectives from lecture 7; ME.21 Create an energy story for the reaction catalyzed by glyceraldehyde-3-phosphate dehydrogenase, that discusses specifically the coupling of a redox reaction to a phosphate transfer.
12. Why: More on the core energy-harvesting reaction in glycolysis. Here we want to get explicitly into the relationship between the redox reaction and the phosphate transfer and the energetics of coupling those two reactions - this part of the story is KEY for understanding how redox is linked intimately to the capture of energy into “high-energy” phosphate bonds.
How to practice: Next, look at the figure below (Figure 4). It breaks down the reaction in Figure 3 into two separate reactions that need to happen in this enzyme active site. One reaction is energetically favorable and the other isn’t. We need to understand how the enzyme couples these reactions together. How does the enzyme act like the waterwheel above to link the energy released by oxidation with the endergonic transfer of an inorganic phosphate onto the carbohydrate?

This exercise helps you practice learning objectives: ME.17 Explain the importance of the reaction catalyzed by glyceraldehyde-3-phosphate dehydrogenase in the harvesting of energy in glycolysis. Use this mechanism and figures to unite lessons from the course: enzymes and catalysts, energy coupling, redox, functional group chemistry, etc.; ME.21 Create an energy story for the reaction catalyzed by glyceraldehyde-3-phosphate dehydrogenase, that discusses specifically the coupling of a redox reaction to a phosphate transfer.
13. Why: More on the core energy-harvesting reaction in glycolysis. In this case we consider the coupling between redox and phosphate transfer from the perspective of reaction coordinate diagrams.
How to practice: The figure below (Figure 6) shows hypotheses that explain the role of the enzyme in coupling from a thermodynamic standpoint. Describe the main energetic difference between an uncoupled scenario and a coupled scenario. Why is the valley in the hypothetical coupled figure (labeled thioester intermediate) shallow compared to the equivalent valley on the left? What does this indicate about where the energy released by the oxidation reaction is going?

This exercise helps you practice learning objectives: ME.20 Be able to interpret figures depicting the mechanism of glyceraldehyde-3-phosphate dehydrogenase and identify key steps in the reaction including the role of a catalytic histidine and the formation of a covalent thioester linkage (including its role in energy transfer).
14. Why: More on the core energy-harvesting reaction in glycolysis. Here we look at the mechanism in the active site. Note how we’ve deconstructed this single reaction from many different viewpoints - using the various exercises above to link this story explicitly to previous ideas we’ve seen already in the course.
How to practice: This next step is hard, but you’re up to it! The figure (Figure 7) shows some of the chemical steps involved in the whole reaction—note it takes several steps. The NAD+/NADH is shown in cyan. The glyceraldehyde-3-phosphate is shown in pink and two R groups of amino acids present in the active site of the enzyme are shown in black. See how we’re back to functional groups and molecular chemistry?
-
- What is the first thing that happens in this reaction?
- What does the enzyme do with the substrate? Identify where the redox reaction takes place and label what got oxidized and what got reduced.
- Now, look at the states right before and right after step 3. Pay particular attention to the relationship between what used to be a substrate (now a product) AND the enzyme. What is different? What changed?
- Now, the intellectually difficult but very satisfying part. After answering those questions, can you explain mechanistically (from a molecular standpoint of bonds breaking/making etc.) the small valley in the right hand panel of Figure 6?
- Can you now provide an explanation for how the enzyme managed to couple energy release from oxidation to the transfer of an inorganic phosphate?

For fun, note that a histidine residue is involved (the ring with two nitrogens). Look up the pKa of histidine’s R group on Google and make a preliminary hypothesis about what pH zone this enzyme function in.
This exercise helps you practice learning objectives: ME.20 Be able to interpret figures depicting the mechanism of glyceraldehyde-3-phosphate dehydrogenase and identify key steps in the reaction including the role of a catalytic histidine and the formation of a covalent thioester linkage (including its role in energy transfer).
PRACTICE EXAM QUESTIONS
Question Q13.1
Q13.1 Using the redox table at right, determine which of the following compounds NADH can theoretically transfer electrons to spontaneously.

- acetoacetate
- β-hydroxybutyrate
- Glutathione reduced form
- FAD+
- glyceraldehyde-3-phosphate
Question Q13.2
Q13.2 Refer to table on the right. The energy derived from the hydrolysis of the phosphate on which of the following compounds can be coupled to the synthesis of ATP?

- Glucose 1-phosphate
- Glucose 6-phosphate
- Creatine phosphate
- Either glucose 1-phosphate or glucose 6-phosphate
- None of these compounds; ATP must be made by an ATP synthase.
Question Q13.3
Q13.3 Which of the following reactions could be energetically coupled to the reaction glucose + Pi → glucose-6-phosphate (+8.0 kcal/mol) to yield a thermodynamically favorable reaction?
- AP → A + Pi (-4 kcal/mol)
- B + Pi → BP (+7 kcal/mol)
- CP → C + Pi (-10 kcal/mol)
- DP → D + Pi(-7kcal/mol)
- E + Pi → EP (+9 kcal/mol)
For the next question refer to the figures on the following pages:
Question Q13.4
Q13.4 Examine the pathway showing the 10 steps in glycolysis and the associated table of ∆G values. The reaction catalyzed by the enzyme _________________ can be practically considered as not reversible under cellular conditions.
- aldolase
- glyceraldehyde-3-phosphate dehydrogenase
- enolase
- hexokinase
- phosphoglycerate kinase






