W2018_Bis2A_Lecture13_reading
( \newcommand{\kernel}{\mathrm{null}\,}\)
Glycolysis: an overview
Organisms, whether unicellular or multicellular, need to find ways of getting at least two key things from their environment: (1) matter or raw materials for maintaining a cell and building new cells and (2) energy to help with the work of staying alive and reproducing. Energy and the raw materials may come from different places. For instance, organisms that primarily harvest energy from sunlight will get raw materials for building biomolecules from sources like CO2. By contract, some organisms rely on red/ox reactions with small molecules and/or reduced metals for energy and get their raw materials for building biomolecules from compounds unconnected to the energy source. Meanwhile, some organisms (including ourselves), have evolved to get energy AND the raw materials for building and cellular maintenance from sometimes associated sources.
Glycolysis is the first metabolic pathway discussed in BIS2A; a metabolic pathway is a series of linked biochemical reactions. Because of its ubiquity in biology, it is hypothesized that glycolysis was probably one of the earliest metabolic pathways to evolve (more on this later). Glycolysis is a ten-step metabolic pathway that is centered on the processing of glucose for both energy extraction from chemical fuel and for the processing of the carbons in glucose into various other biomolecules (some of which are key precursors of many much more complicated biomolecules). Our study of glycolysis will therefore be examined using the precepts outlined in the energy challenge rubric that ask us to formally consider what happens to BOTH matter and energy in this multistep process.
The energy story and design challenge of glycolysis
Our investigation of glycolysis is a good opportunity to examine a biological process using both the energy story and the design challenge rubrics and perspectives.
The design challenge rubric will try to get you to think actively, and broadly and specifically, about why we are studying this pathway—what is so important about it? What "problems" does the evolution of a glycolytic pathway allow life to solve or overcome? We will also want to think about alternate ways to solve the same problems and why they may or may not have evolved. Later, we will examine a hypothesis for how this pathway—and other linked pathways—may have actually evolved, and thinking about alternative strategies for satisfying various constraints will come in handy then.
In the context of the energy story, we will ask you to think about glycolysis as a process from which something can be learned by analyzing what happens to both matter and energy. That is, even though it is a ten-step biochemical pathway, we propose that some insight can be learned by carefully examining the process as a set of matter and energy inputs and outputs, a process with a beginning and an end.
So what is glycolysis? Let's start to find out.

| Enzyme | Step | ΔG/(kJ/mol) | ΔG°'/(kJ/mol) |
|---|---|---|---|
| Hexokinase | 1 | -34 | -16.7 |
| Phosphoglucose isomerase | 2 | -2.9 | 1.67 |
| Phosphofructokinase | 3 | -19 | -14.2 |
| Fructose-bisphosphate aldolase | 4 | -0.23 | 23.9 |
| Triose phosphate isomerase | 5 | 2.4 | 7.56 |
| Glyceraldehyde 3-phosphate dehydrogenase | 6 | -1.29 | 6.30 |
| Phosphoglycerate kinase | 7 | 0.09 | -18.9 |
| Phosphoglycerate mutase | 8 | 0.83 | 4.4 |
| Enolase | 9 | 1.1 | 1.8 |
| Pyruvate kinase | 10 | -23.0 | -31.7 |
Overall, the glycolytic pathway consists of 10 enzyme-catalyzed steps. The primary input into this pathway is a single molecule of glucose, though we will discover that molecules may feed in and out of this pathway at various steps. We will focus our attention on (1) consequences of the overall process, (2) several key reactions that highlight important types of biochemistry and biochemical principles we will want to carry forward to other contexts, and (3) alternative fates of the intermediates and products of this pathway.
Note for reference that glycolysis is an anaerobic process; there is no requirement for molecular oxygen in glycolysis (oxygen gas is not a reactant in any of the chemical reactions in glycolysis). Glycolysis occurs in the cytosol or cytoplasm of cells. For a short (three-minute) overview YouTube video of glycolysis, click here.
First half of glycolysis: energy investment phase
The first few steps of glycolysis are typically referred to as an "energy investment phase" of the pathway. This, however, doesn't make much intuitive sense (in the framework of a design challenge; it's not clear what problem this energy investment solves) if one only looks at glycolysis as an "energy-producing" pathway and until these steps of glycolysis are put into a broader metabolic context. We'll try to build that story as we go, so for now just recall that we mentioned that some of the first steps are often associated with energy investment and ideas like "trapping" and "commitment" that are noted in the figure below.
Step 1 of glycolysis:
The first step in glycolysis, shown below in Figure 2, is glucose being catalyzed by hexokinase, an enzyme with broad specificity that catalyzes the phosphorylation of six-carbon sugars. Hexokinase catalyzes the phosphorylation of glucose, where glucose and ATP are substrates for the reaction, producing a molecule called glucose 6-phosphate and ADP as products.
Figure 2. The first half of glycolysis is called the energy investment phase. In this phase, the cell expends two ATPs into the reactions. Attribution: Marc T. Facciotti (original work)
Suggested discussion
The paragraph above states that the enzyme hexokinase has "broad specificity." This means that it can catalyze reactions with different sugars, not just glucose. From a molecular perspective, can you explain why this might be the case? Does this challenge your conception of enzyme specificity? If you Google the term "enzyme promiscuity" (don't worry; it's safe for work), does this give you a broader appreciation for enzyme selectivity and activity?
The conversion of glucose to the negatively charged glucose 6-phosphate significantly reduces the likelihood that the phosphorylated glucose leaves the cell by diffusion across the hydrophobic interior of the plasma membrane. It also "marks" the glucose in a way that effectively tags it for several different possible fates (see Figure 3).

Figure 3. Note that this figure indicates that glucose 6-phosphate can, depending on cellular conditions, be directed to multiple fates. While it is a component of the glycolytic pathway, it is not only involved in glycolysis but also in the storage of energy as glycogen (colored in cyan) and in the building of various other molecules like nucleotides (colored in red). Source: Marc T. Facciotti (original work)
As Figure 3 indicates, glycolysis is but one possible fate for glucose 6-phosphate (G6P). Depending on cellular conditions, G6P may be diverted to the biosynthesis of glycogen (a form of energy storage), or it may be diverted into the pentose phosphate pathway for the biosynthesis of various biomolecules, including nucleotides. This means that G6P, while involved in the glycolytic pathway, is not solely tagged for oxidation at this phase. Perhaps showing the broader context that this molecule is involved in (in addition to the rationale that tagging glucose with a phosphate decreases the likelihood that it will leave the cell) helps to explain the seemingly contradictory (if you only consider glycolysis as an "energy-producing" process) reason for transferring energy from ATP onto glucose if it is only to be oxidized later—that is, glucose is not only used by the cell for harvesting energy and several other metabolic pathways depend on the transfer of the phosphate group.
Step 2 of glycolysis:
In the second step of glycolysis, an isomerase catalyzes the conversion of glucose 6-phosphate into one of its isomers, fructose 6-phosphate. An isomerase is an enzyme that catalyzes the conversion of a molecule into one of its isomers.
Step 3 of glycolysis:
The third step of glycolysis is the phosphorylation of fructose 6-phosphate, catalyzed by the enzyme phosphofructokinase. A second ATP molecule donates a phosphate to fructose 6-phosphate, producing fructose 1,6-bisphosphate and ADP as products. In this pathway, phosphofructokinase is a rate-limiting enzyme, and its activity is tightly regulated. It is allosterically activated by AMP when the concentration of AMP is high and when it is moderately allosterically inhibited by ATP at the same site. Citrate, a compound we'll discuss soon, also acts as a negative allosteric regulator of this enzyme. In this way, phosphofructokinase monitors or senses molecular indicators of the energy status of the cells and can in response act as a switch that turns on or off the flow of the substrate through the rest of the metabolic pathway depending on whether there is “sufficient” ATP in the system. The conversion of fructose 6-phosphate into fructose 1,6-bisphosphate is sometimes referred to as a commitment step by the cell to the oxidation of the molecule in the rest of the glycolytic pathway by creating a substrate for and helping to energetically drive the next highly endergonic (under standard conditions) step of the pathway.
Suggested discussion
We discussed allosteric regulation of an enzyme in earlier modules but did so in a context where the enzyme was "alone." Now let's consider the enzyme in the context of an extended metabolic pathway(s). Can you now express why allosteric regulation is functionally important and how it can be used to regulate the flow of compounds through a pathway? Try to express yourself.
Step 4 of glycolysis:
In the fourth step in glycolysis, an enzyme, fructose-bisphosphate aldolase, cleaves 1,6-bisphosphate into two three-carbon isomers: dihydroxyacetone phosphate and glyceraldehyde 3-phosphate.
Second half: energy payoff phase
If viewed in the absence of other metabolic pathways, glycolysis has thus far cost the cell two ATP molecules and produced two small, three-carbon sugar molecules: dihydroxyacetone phosphate (DAP) and glyceraldehyde 3-phosphate (G3P). When viewed in a broader context, this investment of energy to produce a variety of molecules that can be used in a variety of other pathways doesn't seem like such a bad investment.
Both DAP and G3P can proceed through the second half of glycolysis. We now examine these reactions.
Figure 4. The second half of glycolysis is called the energy payoff phase. In this phase, the cell gains two ATP and two NADH compounds. At the end of this phase, glucose has become partially oxidized to form pyruvate. Attribution: Marc T. Facciotti (original work).
Step 5 of glycolysis:
In the fifth step of glycolysis, an isomerase transforms the dihydroxyacetone phosphate into its isomer, glyceraldehyde 3-phosphate. The six-carbon glucose has therefore now been converted into two phosphorylated three-carbon molecules of G3P.
Step 6 of glycolysis:
The sixth step is key and one from which we can now leverage our understanding of the several types of chemical reactions that we've studied so far. If you're energy focused, this is finally a step of glycolysis where some of the reduced sugar is oxidized. The reaction is catalyzed by the enzyme glyceraldehyde 3-phosphate dehydrogenase. This enzyme catalyzes a multistep reaction between three substrates—glyceraldehyde 3-phosphate, the cofactor NAD+, and inorganic phosphate (Pi)—and produces three products: 1,3-bisphosphoglycerate, NADH, and H+. One can think of this reaction as two reactions: (1) an oxidation/reduction reaction and (2) a condensation reaction in which an inorganic phosphate is transferred onto a molecule. In this particular case, the red/ox reaction, a transfer of electrons off of G3P and onto NAD+, is exergonic, and the phosphate transfer happens to be endergonic. The net standard free energy change hovers around zero—more on this later. The enzyme here acts as a molecular coupling agent to couple the energetics of the exergonic reaction to that of the endergonic reaction, thus driving both forward. This processes happens through a multistep mechanism in the enzyme's active site and involves the chemical activity of a variety of functional groups.
It is important to note that this reaction depends upon the availability of the oxidized form of the electron carrier, NAD+. If we consider that there is a limiting pool of NAD+, we can then conclude that the reduced form of the carrier (NADH) must be continuously oxidized back into NAD+ in order to keep this step going. If NAD+ is not available, the second half of glycolysis slows down or stops.
Step 7 of glycolysis:
In the seventh step of glycolysis, catalyzed by phosphoglycerate kinase (an enzyme named for the reverse reaction), 1,3-bisphosphoglycerate transfers a phosphate to ADP, forming one molecule of ATP and a molecule of 3-phosphoglycerate. This reaction is exergonic and is also an example of substrate-level phosphorylation.
Possible discussion
If a transfer of a phosphate from 1,3-BPG to ADP is exergonic, what does that say about the free energy of hydrolysis of the phosphate from 1,3-BPG as compared to the free energy of hydrolysis of the terminal phosphate on ATP?
Step 8 of glycolysis:
In the eighth step, the remaining phosphate group in 3-phosphoglycerate moves from the third carbon to the second carbon, producing 2-phosphoglycerate (an isomer of 3-phosphoglycerate). The enzyme catalyzing this step is a mutase (isomerase).
Step 9 of glycolysis:
Enolase catalyzes the ninth step. This enzyme causes 2-phosphoglycerate to lose water from its structure; this is a dehydration reaction, resulting in the formation of a double bond that increases the potential energy in the remaining phosphate bond and produces phosphoenolpyruvate (PEP).
Step 10 of glycolysis:
The last step in glycolysis is catalyzed by the enzyme pyruvate kinase (the enzyme in this case is named for the reverse reaction of pyruvate’s conversion into PEP) and results in the production of a second ATP molecule by substrate-level phosphorylation and the compound pyruvic acid (or its salt form, pyruvate). Many enzymes in enzymatic pathways are named for the reverse reactions, since the enzyme can catalyze both forward and reverse reactions (these may have been described initially by the reverse reaction that takes place in vitro, under non-physiological conditions).
Outcomes of glycolysis
Here are a couple of things to consider:
One of the clear outcomes of glycolysis is the biosynthesis of compounds that can enter into a variety of metabolic pathways. Likewise, compounds coming from other metabolic pathways can feed into glycolysis at various points. So, this pathway can be part of a central exchange for carbon flux within the cell.
If glycolysis is run long enough, the constant oxidation of glucose with NAD+ can leave the cell with a problem: how to regenerate NAD+ from the two molecules of NADH produced. If the NAD+ is not regenerated, all of the cell's NAD will be nearly completely transformed into NADH. So how do cells regenerate NAD+?
Pyruvate is not completely oxidized; there is still some energy to be extracted. How might this happen? Also, what should the cell do with all of that NADH? Is there any energy there to extract?
Strongly suggested discussion/exercise
Can you write an energy story for the overall process of glycolysis? For energy terms, just worry about describing things in terms of whether they are exergonic or endergonic. When I say "overall process," I mean overall process: glucose should be listed on the reactant side of the arrow, and pyruvate should be listed on the product side of the arrow.
Substrate-level phosphorylation (SLP)
The simplest route to synthesize ATP is substrate-level phosphorylation. ATP molecules are generated (that is, regenerated from ADP) as a direct result of a chemical reaction that occurs in catabolic pathways. A phosphate group is removed from an intermediate reactant in the pathway, and the free energy of the reaction is used to add the third phosphate to an available ADP molecule, producing ATP. This very direct method of phosphorylation is called substrate-level phosphorylation. It can be found in a variety of catabolic reactions, most notably in two specific reactions in glycolysis (which we will discuss specifically later). Suffice it to say that what is required is a high-energy intermediate whose oxidation is sufficient to drive the synthesis of ATP.
In this reaction, the reactants are a phosphorylated carbon compound called G3P (from step 6 of glycolysis) and an ADP molecule, and the products are 1,3-BPG and ATP. The transfer of the phosphate from G3P to ADP to form ATP in the active site of the enzyme is substrate-level phosphorylation. This occurs twice in glycolysis and once in the TCA cycle (for a subsequent reading).
Fermentation and regeneration of NAD+
Section summary
This section discusses the process of fermentation. Due to the heavy emphasis in this course on central carbon metabolism, the discussion of fermentation understandably focuses on the fermentation of pyruvate. Nevertheless, some of the core principles that we cover in this section apply equally well to the fermentation of many other small molecules.
The "purpose" of fermentation
The oxidation of a variety of small organic compounds is a process that is utilized by many organisms to garner energy for cellular maintenance and growth. The oxidation of glucose via glycolysis is one such pathway. Several key steps in the oxidation of glucose to pyruvate involve the reduction of the electron/energy shuttle NAD+ to NADH. You were already asked to figure out what options the cell might reasonably have to reoxidize the NADH to NAD+ in order to avoid consuming the available pools of NAD+ and to thus avoid stopping glycolysis. Put differently, during glycolysis, cells can generate large amounts of NADH and slowly exhaust their supplies of NAD+. If glycolysis is to continue, the cell must find a way to regenerate NAD+, either by synthesis or by some form of recycling.
In the absence of any other process—that is, if we consider glycolysis alone—it is not immediately obvious what the cell might do. One choice is to try putting the electrons that were once stripped off of the glucose derivatives right back onto the downstream product, pyruvate, or one of its derivatives. We can generalize the process by describing it as the returning of electrons to the molecule that they were once removed, usually to restore pools of an oxidizing agent. This, in short, is fermentation. As we will discuss in a different section, the process of respiration can also regenerate the pools of NAD+ from NADH. Cells lacking respiratory chains or in conditions where using the respiratory chain is unfavorable may choose fermentation as an alternative mechanism for garnering energy from small molecules.
An example: lactic acid fermentation
An everyday example of a fermentation reaction is the reduction of pyruvate to lactate by the lactic acid fermentation reaction. This reaction should be familiar to you: it occurs in our muscles when we exert ourselves during exercise. When we exert ourselves, our muscles require large amounts of ATP to perform the work we are demanding of them. As the ATP is consumed, the muscle cells are unable to keep up with the demand for respiration, O2 becomes limiting, and NADH accumulates. Cells need to get rid of the excess and regenerate NAD+, so pyruvate serves as an electron acceptor, generating lactate and oxidizing NADH to NAD+. Many bacteria use this pathway as a way to complete the NADH/NAD+ cycle. You may be familiar with this process from products like sauerkraut and yogurt. The chemical reaction of lactic acid fermentation is the following:
Pyruvate + NADH ↔ lactic acid + NAD+

Figure 1. Lactic acid fermentation converts pyruvate (a slightly oxidized carbon compound) to lactic acid. In the process, NADH is oxidized to form NAD+. Attribution: Marc T. Facciotti (original work)
Energy story for the fermentation of pyruvate to lactate
An example (if a bit lengthy) energy story for lactic acid fermentation is the following:
The reactants are pyruvate, NADH, and a proton. The products are lactate and NAD+. The process of fermentation results in the reduction of pyruvate to form lactic acid and the oxidation of NADH to form NAD+. Electrons from NADH and a proton are used to reduce pyruvate into lactate. If we examine a table of standard reduction potential, we see under standard conditions that a transfer of electrons from NADH to pyruvate to form lactate is exergonic and thus thermodynamically spontaneous. The reduction and oxidation steps of the reaction are coupled and catalyzed by the enzyme lactate dehydrogenase.
A second example: alcohol fermentation
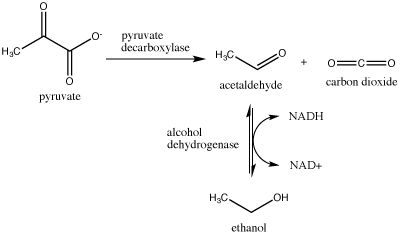
Another familiar fermentation process is alcohol fermentation, which produces ethanol, an alcohol. The alcohol fermentation reaction is the following:
Figure 2. Ethanol fermentation is a two-step process. Pyruvate (pyruvic acid) is first converted into carbon dioxide and acetaldehyde. The second step converts acetaldehyde to ethanol and oxidizes NADH to NAD+. Attribution: Marc T. Facciotti (original work)
In the first reaction, a carboxyl group is removed from pyruvic acid, releasing carbon dioxide as a gas (some of you may be familiar with this as a key component of various beverages). The second reaction removes electrons from NADH, forming NAD+ and producing ethanol (another familiar compound—usually in the same beverage) from the acetaldehyde, which accepts the electrons.
Suggested discussion
Write a complete energy story for alcohol fermentation. Propose possible benefits of this type of fermentation for the single-celled yeast organism.
Fermentation pathways are numerous
While the lactic acid fermentation and alcohol fermentation pathways described above are examples, there are many more reactions (too numerous to go over) that Nature has evolved to complete the NADH/NAD+ cycle. It is important that you understand the general concepts behind these reactions. In general, cells try to maintain a balance or constant ratio between NADH and NAD+; when this ratio becomes unbalanced, the cell compensates by modulating other reactions to compensate. The only requirement for a fermentation reaction is that it uses a small organic compound as an electron acceptor for NADH and regenerates NAD+. Other familiar fermentation reactions include ethanol fermentation (as in beer and bread), propionic fermentation (it's what makes the holes in Swiss cheese), and malolactic fermentation (it's what gives Chardonnay its more mellow flavor—the more conversion of malate to lactate, the softer the wine). In Figure 3, you can see a large variety of fermentation reactions that various bacteria use to reoxidize NADH to NAD+. All of these reactions start with pyruvate or a derivative of pyruvate metabolism, such as oxaloacetate or formate. Pyruvate is produced from the oxidation of sugars (glucose or ribose) or other small, reduced organic molecules. It should also be noted that other compounds can be used as fermentation substrates besides pyruvate and its derivatives. These include methane fermentation, sulfide fermentation, or the fermentation of nitrogenous compounds such as amino acids. You are not expected to memorize all of these pathways. You are, however, expected to recognize a pathway that returns electrons to products of the compounds that were originally oxidized to recycle the NAD+/NADH pool and to associate that process with fermentation.
Figure 3. This figure shows various fermentation pathways using pyruvate as the initial substrate. In the figure, pyruvate is reduced to a variety of products via different and sometimes multistep (dashed arrows represent possible multistep processes) reactions. All details are deliberately not shown. The key point is to appreciate that fermentation is a broad term not solely associated with the conversion of pyruvate to lactic acid or ethanol. Source: Marc T. Facciotti (original work)
A note on the link between substrate-level phosphorylation and fermentation
Fermentation occurs in the absence of molecular oxygen (O2). It is an anaerobic process. Notice there is no O2 in any of the fermentation reactions shown above. Many of these reactions are quite ancient, hypothesized to be some of the first energy-generating metabolic reactions to evolve. This makes sense if we consider the following:
- The early atmosphere was highly reduced, with little molecular oxygen readily available.
- Small, highly reduced organic molecules were relatively available, arising from a variety of chemical reactions.
- These types of reactions, pathways, and enzymes are found in many different types of organisms, including bacteria, archaea, and eukaryotes, suggesting these are very ancient reactions.
- The process evolved long before O2 was found in the environment.
- The substrates, highly reduced, small organic molecules, like glucose, were readily available.
- The end products of many fermentation reactions are small organic acids, produced by the oxidation of the initial substrate.
- The process is coupled to substrate-level phosphorylation reactions. That is, small, reduced organic molecules are oxidized, and ATP is generated by first a red/ox reaction followed by the substrate-level phosphorylation.
- This suggests that substrate-level phosphorylation and fermentation reactions coevolved.
Suggested discussion
If the hypothesis is correct that substrate-level phosphorylation and fermentation reactions co-evolved and were the first forms of energy metabolism that cells used to generate ATP, then what would be the consequences of such reactions over time? What if these were the only forms of energy metabolism available over hundreds of thousands of years? What if cells were isolated in a small, closed environment? What if the small, reduced substrates were not being produced at the same rate of consumption during this time?
Consequences of fermentation
Imagine a world where fermentation is the primary mode for extracting energy from small molecules. As populations thrive, they reproduce and consume the abundance of small, reduced organic molecules in the environment, producing acids. One consequence is the acidification (decrease in pH) of the environment, including the internal cellular environment. This can be disruptive, since changes in pH can have a profound influence on the function and interactions among various biomolecules. Therefore, mechanisms needed to evolve that could remove the various acids. Fortunately, in an environment rich in reduced compounds, substrate-level phosphorylation and fermentation can produce large quantities of ATP.
It is hypothesized that this scenario was the beginning of the evolution of the F0F1-ATPase, a molecular machine that hydrolyzes ATP and translocates protons across the membrane (we'll see this again in the next section). With the F0F1-ATPase, the ATP produced from fermentation could now allow for the cell to maintain pH homeostasis by coupling the free energy of hydrolysis of ATP to the transport of protons out of the cell. The downside is that cells are now pumping all of these protons into the environment, which will now start to acidify.
Suggested discussion
If the hypothesis is correct that the F0F1-ATPase also co-evolved with substrate-level phosphorylation and fermentation reactions, then what would happen over time to the environment? While small, reduced organic compounds may have been initially abundant, if fermentation "took off" at some point, then the reduced compounds would run out and ATP might then become scarce as well. That's a problem. Thinking with the design challenge rubric in mind, define the problem(s) facing the cell in this hypothesized environment. What are other potential mechanisms or ways Nature could overcome the problem(s)?
Oxidation of Pyruvate and the TCA Cycle
Overview of Pyruvate Metabolism and the TCA Cycle
Under appropriate conditions, pyruvate can be further oxidized. One of the most studied oxidation reactions involving pyruvate is a two-part reaction involving NAD+ and molecule called co-enzyme A, often abbreviated simply as "CoA". This reaction oxidizes pyruvate, leads to a loss of one carbon via decarboxylation, and creates a new molecule called acetyl-CoA. The resulting acetyl-CoA can enter several pathways for the biosynthesis of larger molecules or it can be routed to another pathway of central metabolism called the Citric Acid Cycle, sometimes also called the Krebs Cycle, or Tricarboxylic Acid (TCA) Cycle. Here the remaining two carbons in the acetyl group can either be further oxidized or serve again as precursors for the construction of various other molecules. We discuss these scenarios below.
The different fates of pyruvate and other end products of glycolysis
The glycolysis module left off with the end-products of glycolysis: 2 pyruvate molecules, 2 ATPs and 2 NADH molecules. This module and the module on fermentation explore what the cell can do with the pyruvate, ATP and NADH that were generated.
The fates of ATP and NADH
In general, ATP can be used for or coupled to a variety of cellular functions including biosynthesis, transport, replication etc. We will see many such examples throughout the course.
What to do with the NADH however, depends on the conditions under which the cell is growing. In some cases, the cell will opt to rapidly recycle NADH back into to NAD+. This occurs through a process called fermentation in which the electrons initially taken from the glucose derivatives are returned to more downstream products via another red/ox transfer (described in more detail in the module on fermentation). Alternatively, NADH can be recycled back into NAD+ by donating electrons to something known as an electron transport chain (this is covered in the module on respiration and electron transport).
The fate of cellular pyruvate
- Pyruvate can be used as a terminal electron acceptor (either directly or indirectly) in fermentation reactions, and is discussed in the fermentation module.
- Pyruvate could be secreted from the cell as a waste product.
- Pyruvate could be further oxidized to extract more free energy from this fuel.
- Pyruvate can serve as a valuable intermediate compound linking some of the core carbon processing metabolic pathways
The further oxidation of pyruvate
In respiring bacteria and archaea, the pyruvate is further oxidized in the cytoplasm. In aerobically respiring eukaryotic cells, the pyruvate molecules produced at the end of glycolysis are transported into mitochondria, which are sites of cellular respiration and house oxygen consuming electron transport chains (ETC in module on respiration and electron transport). Organisms from all three domains of life share similar mechanisms to further oxidize the pyruvate to CO2. First pyruvate is decarboxylated and covalently linked to co-enzyme A via a thioester linkage to form the molecule known as acetyl-CoA. While acetyl-CoA can feed into multiple other biochemical pathways we now consider its role in feeding the circular pathway known as the Tricarboxylic Acid Cycle, also referred to as the TCA cycle, the Citric Acid Cycle or the Krebs Cycle. This process is detailed below.
Conversion of Pyruvate into Acetyl-CoA
In a multi-step reaction catalyzed by the enzyme pyruvate dehydrogenase, pyruvate is oxidized by NAD+, decarboxylated, and covalently linked to a molecule of co-enzyme A via a thioester bond. The release of the carbon dioxide is important here, this reaction often results in a loss of mass from the cell as the CO2 will diffuse or be transported out of the cell and become a waste product. In addition, one molecule of NAD+ is reduced to NADH during this process per molecule of pyruvate oxidized. Remember: there are two pyruvate molecules produced at the end of glycolysis for every molecule of glucose metabolized; thus, if both of these pyruvate molecules are oxidized to acetyo-CoA two of the original six carbons will be converted to waste.
Suggested discussion
We have already discussed the formation of a thioester bond in another unit and lecture. Where was this specifically? What was the energetic significance of this bond? What are the similarities and differences between this example (formation of thioester with CoA) and the previous example of this chemistry?

Suggested discussion
Describe the flow and transfer of energy in this reaction using good vocabulary - (e.g. reduced, oxidized, red/ox, endergonic, exergonic, thioester, etc. etc.). You can peer edit - someone can start a description, another person can make it better, another person can improve it more etc. . .
In the presence of a suitable terminal electron acceptor, acetyl CoA delivers (exchanges a bond) its acetyl group to a four-carbon molecule, oxaloacetate, to form citrate (designated the first compound in the cycle). This cycle is called by different names: the citric acid cycle (for the first intermediate formed—citric acid, or citrate), the TCA cycle (since citric acid or citrate and isocitrate are tricarboxylic acids), and the Krebs cycle, after Hans Krebs, who first identified the steps in the pathway in the 1930s in pigeon flight muscles.
The Tricarboxcylic Acid (TCA) Cycle
In bacteria and archaea reactions in the TCA cycle typically happen in the cytosol. In eukaryotes, the TCA cycle takes place in the matrix of mitochondria. Almost all (but not all) of the enzymes of the TCA cycle are water soluble (not in the membrane), with the single exception of the enzyme succinate dehydrogenase, which is embedded in the inner membrane of the mitochondrion (in eukaryotes). Unlike glycolysis, the TCA cycle is a closed loop: the last part of the pathway regenerates the compound used in the first step. The eight steps of the cycle are a series of red/ox, dehydration, hydration, and decarboxylation reactions that produce two carbon dioxide molecules, one ATP, and reduced forms of NADH and FADH2.
Figure 2. In the TCA cycle, the acetyl group from acetyl CoA is attached to a four-carbon oxaloacetate molecule to form a six-carbon citrate molecule. Through a series of steps, citrate is oxidized, releasing two carbon dioxide molecules for each acetyl group fed into the cycle. In the process, three NAD+ molecules are reduced to NADH, one FAD+ molecule is reduced to FADH2, and one ATP or GTP (depending on the cell type) is produced (by substrate-level phosphorylation). Because the final product of the TCA cycle is also the first reactant, the cycle runs continuously in the presence of sufficient reactants.
Attribution: “Yikrazuul”/Wikimedia Commons (modified)
Note
We are explicitly making reference to eukaryotes, bacteria and archaea when we discuss the location of the TCA cycle because many beginning students of biology tend to exclusively associate the TCA cycle with mitochondria. Yes, the TCA cycle occurs in the mitochondria of eukaryotic cells. However, this pathway is not exclusive to eukaryotes; it occurs in bacteria and archaea too!
Steps in the TCA Cycle
Step 1:
The first step of the cycle is a condensation reaction involving the two-carbon acetyl group of acetyl-CoA with one four-carbon molecule of oxaloacetate. The products of this reaction are the six-carbon molecule citrate and free co-enzyme A. This step is considered irreversible because it is so highly exergonic. Moreover, the rate of this reaction is controlled through negative feedback by ATP. If ATP levels increase, the rate of this reaction decreases. If ATP is in short supply, the rate increases. If not already, the reason will become evident shortly.
Step 2:
In step two, citrate loses one water molecule and gains another as citrate is converted into its isomer, isocitrate.
Step 3:
In step three, isocitrate is oxidized by NAD+ and decarboxylated. Keep track of the carbons! This carbon now more than likely leaves the cell as waste and is no longer available for building new biomolecules. The oxidation of isocitrate therefore produces a five-carbon molecule, α-ketoglutarate, a molecule of CO2 and NADH. This step is also regulated by negative feedback from ATP and NADH, and via positive feedback from ADP.
Step 4:
Step 4 is catalyzed by the enzyme succinate dehydrogenase. Here, α-ketoglutarate is further oxidized by NAD+. This oxidation again leads to a decarboxylation and thus the loss of another carbon as waste. So far two carbons have come into the cycle from acetyl-CoA and two have left as CO2. At this stage, there is no net gain of carbons assimilated from the glucose molecules that are oxidized to this stage of metabolism. Unlike the previous step however succinate dehydrogenase - like pyruvate dehydrogenase before it - couples the free energy of the exergonic red/ox and decarboxylation reaction to drive the formation of a thioester bond between the substrate co-enzyme A and succinate (what is left after the decarboxylation). Succinate dehydrogenase is regulated by feedback inhibition of ATP, succinyl-CoA, and NADH.
Suggested discussion
We have seen several steps in this and other pathways that are regulated by allosteric feedback mechanisms. Is there something(s) in common about these steps in the TCA cycle? Why might these be good steps to regulate?
Suggested discussion
The thioester bond has reappeared! Use the terms we've been learning (e.g. reduction, oxidation, coupling, exergonic, endergonic etc.) to describe the formation of this bond and below its hydrolysis.
Step 5:
In step five, a substrate level phosphorylation event occurs. Here an inorganic phosphate (Pi) is added to GDP or ADP to form GTP (an ATP equivalent for our purposes) or ATP. The energy that drives this substrate level phosphorylation event comes from the hydrolysis of the CoA molecule from succinyl~CoA to form succinate. Why is either GTP or ATP produced? In animal cells there are two isoenzymes (different forms of an enzyme that carries out the same reaction), for this step, depending upon the type of animal tissue in which those cells are found. One isozyme is found in tissues that use large amounts of ATP, such as heart and skeletal muscle. This isozyme produces ATP. The second isozyme of the enzyme is found in tissues that have a large number of anabolic pathways, such as liver. This isozyme produces GTP. GTP is energetically equivalent to ATP; however, its use is more restricted. In particular, the process of protein synthesis primarily uses GTP. Most bacterial systems produce GTP in this reaction.
Step 6:
Step six is another red/ox reactions in which succinate is oxidized by FAD+ into fumarate. Two hydrogen atoms are transferred to FAD+, producing FADH2. The difference in reduction potential between the fumarate/succinate and NAD+/NADH half reactions is insufficient to make NAD+ a suitable reagent for oxidizing succinate with NAD+ under cellular conditions. However, the difference in reduction potential with the FAD+/FADH2 half reaction is adequate to oxidize succinate and reduce FAD+. Unlike NAD+, FAD+ remains attached to the enzyme and transfers electrons to the electron transport chain directly. This process is made possible by the localization of the enzyme catalyzing this step inside the inner membrane of the mitochondrion or plasma membrane (depending on whether the organism in question is eukaryotic or not).
Step 7:
Water is added to fumarate during step seven, and malate is produced. The last step in the citric acid cycle regenerates oxaloacetate by oxidizing malate with NAD+. Another molecule of NADH is produced in the process.
Summary
Note that this process (oxidation of pyruvate to Acetyl-CoA followed by one "turn" of the TCA cycle) completely oxidizes 1 molecule of pyruvate, a 3 carbon organic acid, to 3 molecules of CO2. Overall 4 molecules of NADH, 1 molecule of FADH2, and 1 molecule of GTP (or ATP) are also produced. For respiring organisms this is a significant mode of energy extraction, since each molecule of NADH and FAD2 can feed directly into the electron transport chain, and as we will soon see, the subsequent red/ox reactions that are driven by this process will indirectly power the synthesis of ATP. The discussion so far suggests that the TCA cycle is primarily an energy extracting pathway; evolved to extract or convert as much potential energy from organic molecules to a form that cells can use, ATP (or the equivalent) or an energized membrane. However, - and let us not forget - the other important outcome of evolving this pathway is the ability to produce several precursor or substrate molecules necessary for various catabolic reactions (this pathway provides some of the early building blocks to make bigger molecules). As we will discuss below, there is a strong link between carbon metabolism and energy metabolism.
Exercise
TCA Energy Stories
Work on building some energy stories yourself
There are a few interesting reactions that involve large transfers of energy and rearrangements of matter. Pick a few. Rewrite a reaction in your notes, and practice constructing an energy story. You now have the tools to discuss the energy redistribution in the context of broad ideas and terms like exergonic and endergonic. You also have the ability to begin discussing mechanism (how these reactions happen) by invoking enzyme catalysts. See your instructor and/or TA and check with you classmates to self-test on how you're doing.
Connections to Carbon Flow
One hypothesis that we have started exploring in this reading and in class is the idea that "central metabolism" evolved as a means of generating carbon precursors for catabolic reactions. Our hypothesis also states that as cells evolved, these reactions became linked into pathways: glycolysis and the TCA cycle, as a means to maximize their effectiveness for the cell. We can postulate that a side benefit to evolving this metabolic pathway was the generation of NADH from the complete oxidation of glucose - we saw the beginning of this idea when we discussed fermentation. We have already discussed how glycolysis not only provides ATP from substrate level phosphorylation, but also yields a net of 2 NADH molecules and 6 essential precursors: glucose-6-P, fructose-6-P, 3-phosphoglycerate, phosphoenolpyruvate, and of course, pyruvate. While ATP can be used by the cell directly as an energy source, NADH posses a problem and must be recycled back into NAD+, to keep the pathway in balance. As we see in detail in the fermentation module, the most ancient way cells deal with this problem is to use fermentation reactions to regenerate NAD+.
During the process of pyruvate oxidation via the TCA cycle 4 additional essential precursors are formed: acetyl~CoA, α-ketoglutarate, oxaloacetate, and succinyl~CoA. Three molecules of CO2 are lost and this represents a net loss of mass for the cell. These precursors, however, are substrates for a variety of catabolic reactions including the production of amino acids, fatty acids, and various co-factors, such as heme. This means that the rate of reactions through the TCA cycle will be sensitive to the concentrations of each metabolic intermediate (more on the thermodynamics in class). A metabolic intermediate is a compound that is produced by one reaction (a product) and then acts as a substrate for the next reaction. This also means that metabolic intermediates, in particular the 4 essential precursors, can be removed at any time for catabolic reactions, if there is a demand, changing the thermodynamics of the cycle.
Not all cells have a functional TCA cycle
Since all cells require the ability of make these precursor molecules, one might expect that all organisms would have a fully functional TCA cycle. In fact, the cells of many organisms DO NOT have all of the enzymes required to form a complete cycle - all cells, however, DO have the capability of making the 4 TCA cycle precursors noted in the previous paragraph. How can the cells make precursors and not have a full cycle? Remember that most of these reactions are freely reversible, so, if NAD+ is required to for the oxidation of pyruvate or acetyl~CoA, then the reverse reactions would require NADH. This process is often referred to as the reductive TCA cycle. To drive these reactions in reverse (with respect to the direction discussed above) requires energy, in this case carried by ATP and NADH. If you get ATP and NADH driving a pathway one direction, it stands to reason that driving it in reverse will require ATP and NADH as "inputs". So, organisms that do not have a full cycle can still make the 4 key metabolic precursors by using previously extracted energy and electrons (ATP and NADH) to drive some key steps in reverse.
Suggested discussion
Why might some organisms not have evolved a fully oxidative TCA cycle? Remember, cells need to keep a balance in the NAD+ to NADH ratio as well as the [ATP]/[AMP]/[ADP] ratios.
Additional Links
Here are some additional links to videos and pages that you may find useful.
Chemwiki Links
- Chemwiki TCA cycle - link down until key content corrections are made to the resource