5: Amino Acid Structure, Function
( \newcommand{\kernel}{\mathrm{null}\,}\)
I.  alpha amino acids - 20 different ones are used in protein synthesis. The properties page also links to
alpha amino acids - 20 different ones are used in protein synthesis. The properties page also links to  3-D structure views..
3-D structure views..
IV. Peptide bond between amino acids.
The reverse of the pictured reaction is actually strongly energetically favorable, but the high activation energy makes peptide bonds stable under normal conditions.
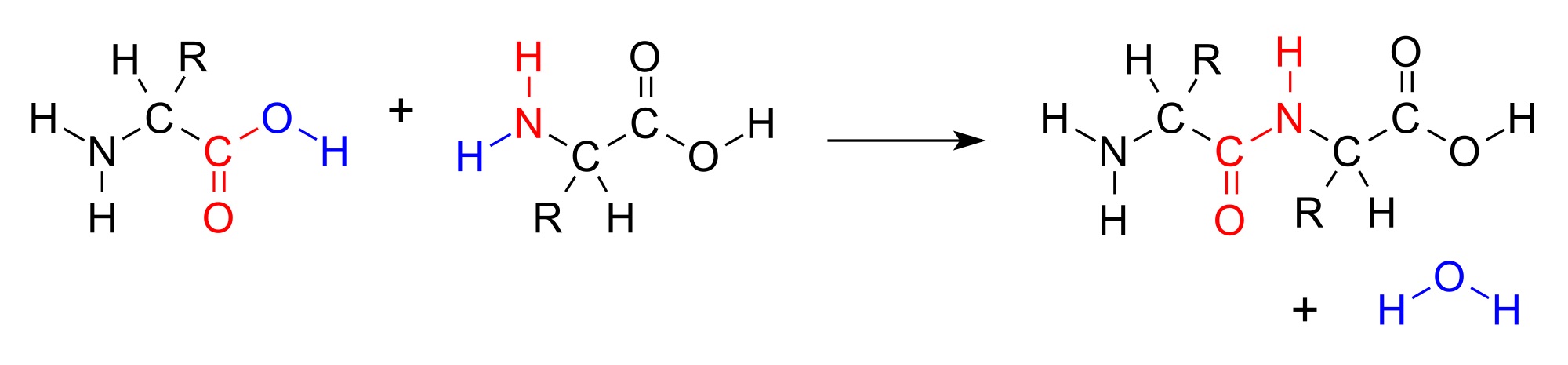
The formation of the peptide bond eliminates the acid/base properties of the involved alpha carboxyl and alpha amino groups. Chains of amino acids connected by peptide bonds are called "peptides", "oligopeptides" or "polypeptides" depending on length. Short peptides can include their length in the name, e. g.: dipeptide, tripeptide, tetrapeptide, pentapeptide, etc.
Note that the chains have directionality. One end will have an amino group - and this is referred to as the "amino terminus" or "N-terminus". The other end will have a carboxyl group and is called the "carboxyl terminus" or "C-terminus".
A. Acid hydrolysis at high temperature and pressure.
(note: also hydrolyses side chain peptide/amide bonds in asparagine and glutamine to produce aspartate and glutamate)
B. Ion exchange chromatography.( here is one schematic animation of the method)
here is one schematic animation of the method)
Contributors
Charles S. Gasser (Department of Molecular & Cellular Biology; UC Davis)

