4.2: Classifying Eukaryotic Microbes and Examples
- Page ID
- 75832
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Identify challenges associated with classifying microbial eukaryotes
- Explain the taxonomic scheme used for microbial eukaryotes
- Give examples of infections caused by microbial eukaryotes
Eukaryotic microbes are an extraordinarily diverse group, including species with a wide range of life cycles, morphological specializations, and nutritional needs. Although more diseases are caused by viruses and bacteria than by microscopic eukaryotes, these eukaryotes are responsible for some diseases of great public health importance. Although it may seem surprising, parasitic worms are included within the study of microbiology because identification depends on observation of microscopic adult worms or eggs. Even in developed countries, these worms are important parasites of humans and of domestic animals. There are fewer fungal pathogens, but these are important causes of illness, as well. On the other hand, fungi have been important in producing antimicrobial substances such as penicillin. In this chapter, we will examine characteristics of protists, worms, and fungi while considering their roles in causing disease.
Characteristics of Protists
The word protist is a historical term that is now used informally to refer to a diverse group of microscopic eukaryotic organisms. It is not considered a formal taxonomic term because the organisms it describes do not have a shared evolutionary origin, termed polyphyletic. Since the current taxonomy is based on evolutionary history (as determined by biochemistry, morphology, and genetics), protists are scattered across many different taxonomic groups within the domain. Historically, the protists were informally grouped into the “animal-like” protozoans, the “plant-like” algae, and the “fungus-like” protists such as water molds. These three groups of protists differ greatly in terms of their basic characteristics. For example, algae are photosynthetic organisms that can be unicellular or multicellular. Protozoa, on the other hand, are nonphotosynthetic, motile organisms that are always unicellular. Other informal terms may also be used to describe various groups of protists. For example, microorganisms that drift or float in water, moved by currents, are referred to as plankton. Types of plankton include zooplankton, which are motile and nonphotosynthetic, and phytoplankton, which are photosynthetic.
Protozoans
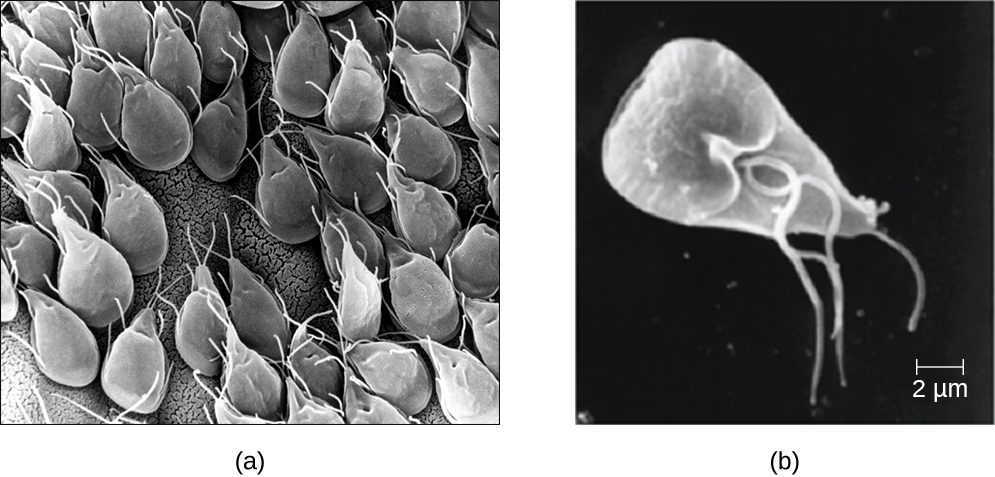
Protozoans inhabit a wide variety of habitats, both aquatic and terrestrial. Protozoans are heterotrophic. Many are free-living, while others are parasitic, carrying out a life cycle within a host or hosts and potentially causing illness. There are also beneficial symbionts that provide metabolic services to their hosts. During the feeding and growth part of their life cycle, they are called trophozoites; these feed on small particulate food sources such as bacteria. For example, the protozoal disease malaria was responsible for 584,000 deaths worldwide (primarily children in Africa) in 2013, according to the World Health Organization (WHO). The protist parasite Giardia causes a diarrheal illness (giardiasis) that is easily transmitted through contaminated water supplies. In the United States, Giardia is the most common human intestinal parasite (Figure \(\PageIndex{2}\)).
The genus Entamoeba includes commensal or parasitic species, including the medically important E. histolytica, which is transmitted by cysts in feces and is the primary cause of amoebic dysentery. The notorious “brain-eating amoeba,” Naegleria fowleri, is a deadly parasite is found in warm, fresh water and causes primary amoebic meningoencephalitis (PAM). Another member of this group is Acanthamoeba, which can cause keratitis (corneal inflammation) and blindness.
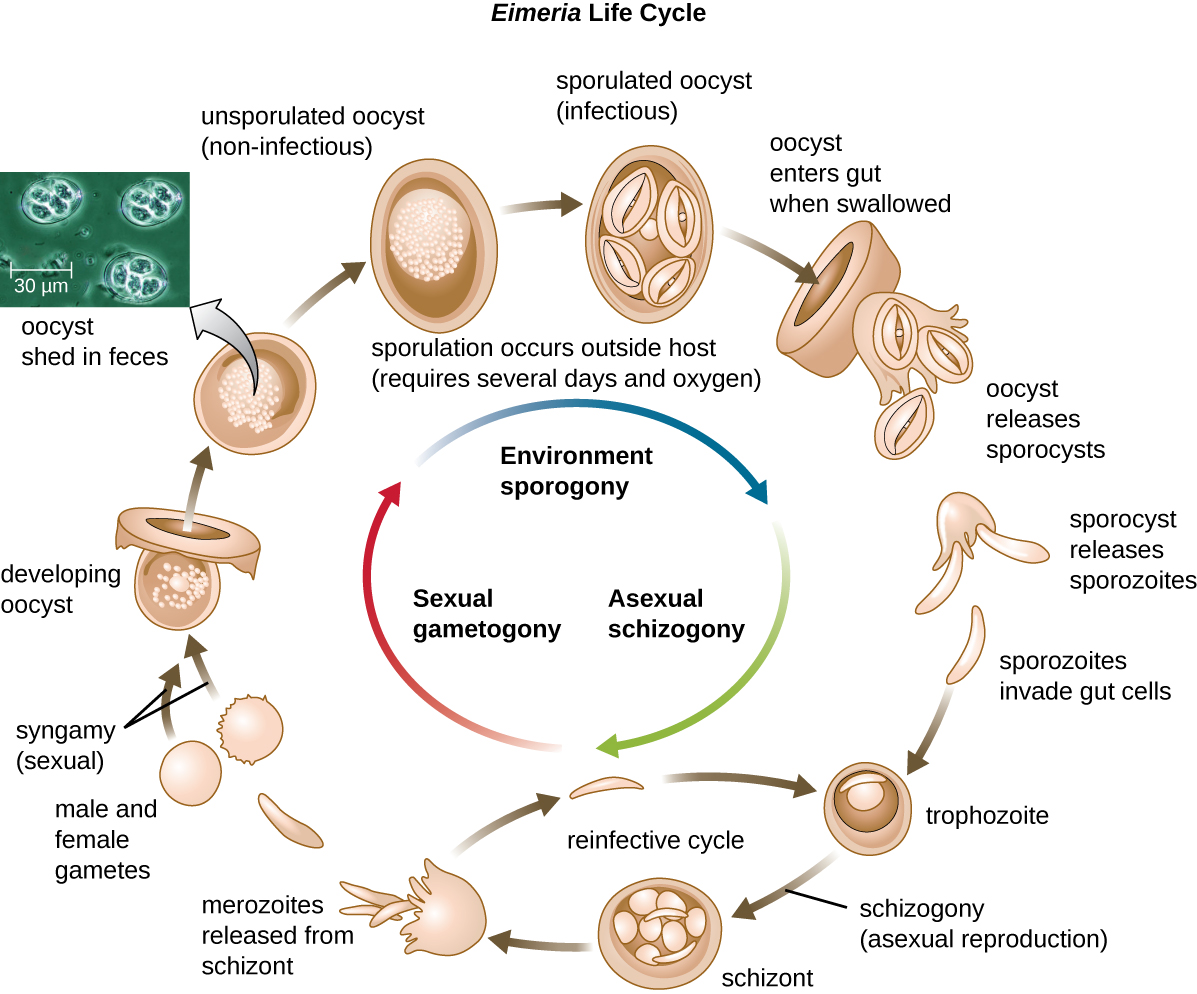
While some types of protozoa exist exclusively in the trophozoite form, others can develop from trophozoite to an encapsulated cyst stage when environmental conditions are too harsh for the trophozoite. A cyst is a cell with a protective wall, and the process by which a trophozoite becomes a cyst is called encystment. When conditions become more favorable, these cysts are triggered by environmental cues to become active again through excystment. One protozoan genus capable of encystment is Eimeria, which includes some human and animal pathogens. Figure \(\PageIndex{3}\) illustrates the life cycle of Eimeria.
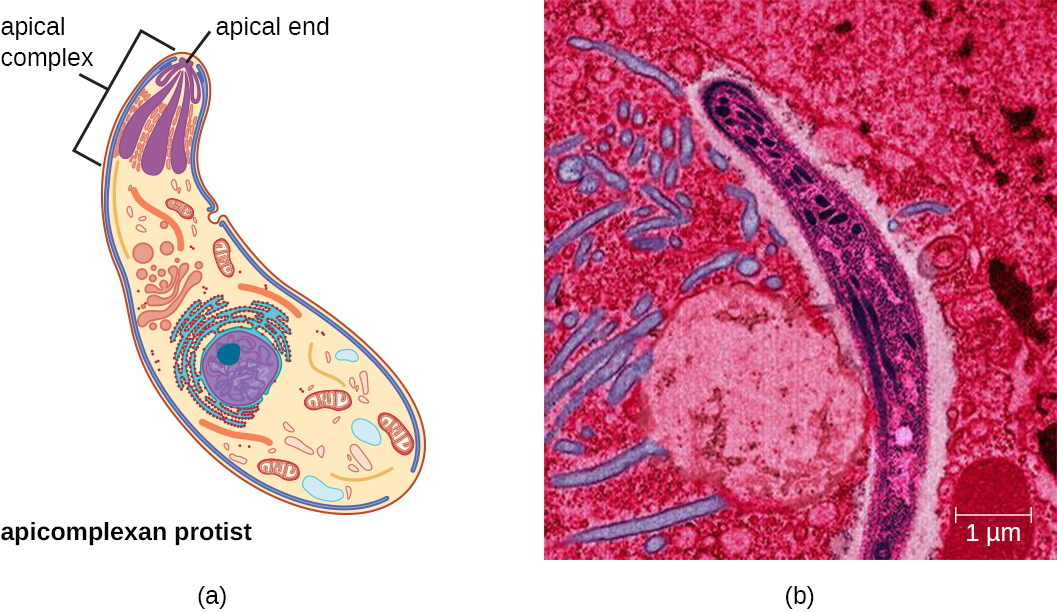
The apicomplexans are intra- or extracellular parasites that have an apical complex at one end of the cell. The apical complex is a concentration of organelles, vacuoles, and microtubules that allows the parasite to enter host cells (Figure \(\PageIndex{4}\)). Apicomplexans have complex life cycles that include an infective sporozoite that undergoes schizogony to make many merozoites. Many are capable of infecting a variety of animal cells, from insects to livestock to humans, and their life cycles often depend on transmission between multiple hosts. The genus Plasmodium is an example of this group.
Another example is Toxoplasma gondii causes toxoplasmosis and can be transmitted from cat feces, unwashed fruit and vegetables, or from undercooked meat. Because toxoplasmosis can be associated with serious birth defects, pregnant women need to be aware of this risk and use caution if they are exposed to the feces of potentially infected cats. A national survey found the frequency of individuals with antibodies for toxoplasmosis (and thus who presumably have a current latent infection) in the United States to be 11%. Rates are much higher in other countries, including some developed countries.1 There is also evidence and a good deal of theorizing that the parasite may be responsible for altering infected humans’ behavior and personality traits.2
Fungi-like protists
The Eumycetozoa are an unusual group of organisms called slime molds, which have previously been classified as animals, fungi, and plants (Figure \(\PageIndex{5}\)). Slime molds can be divided into two types: cellular slime molds and plasmodial slime molds. The cellular slime molds exist as individual amoeboid cells that periodically aggregate into a mobile slug. The aggregate then forms a fruiting body that produces haploid spores. Plasmodial slime molds exist as large, multinucleate amoeboid cells that form reproductive stalks to produce spores that divide into gametes. One cellular slime mold, Dictyostelium discoideum, has been an important study organism for understanding cell differentiation, because it has both single-celled and multicelled life stages, with the cells showing some degree of differentiation in the multicelled form.

Öomycetes have similarities to fungi and were once classified with them. They are also called water molds. However, they differ from fungi in several important ways. Öomycetes have cell walls of cellulose (unlike the chitinous cell walls of fungi) and they are generally diploid, whereas the dominant life forms of fungi are typically haploid. Phytophthora, the plant pathogen found in the soil that caused the Irish potato famine, is classified within this group (Figure \(\PageIndex{6}\)).

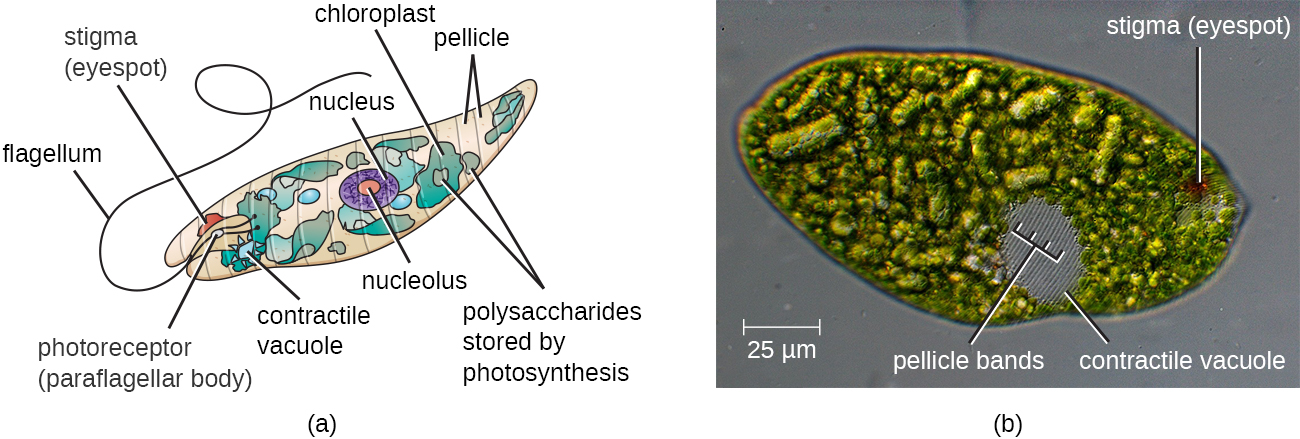
The Euglenozoa are common in the environment and include photosynthetic and nonphotosynthetic species. While some are photosynthesic these are not considered algae because they feed and are motile. Members of the genus Euglena are typically not pathogenic. Their cells have two flagella, a pellicle, a stigma (eyespot) to sense light, and chloroplasts for photosynthesis (Figure \(\PageIndex{7}\)). The pellicle of Euglena is made of a series of protein bands surrounding the cell; it supports the cell membrane and gives the cell shape.
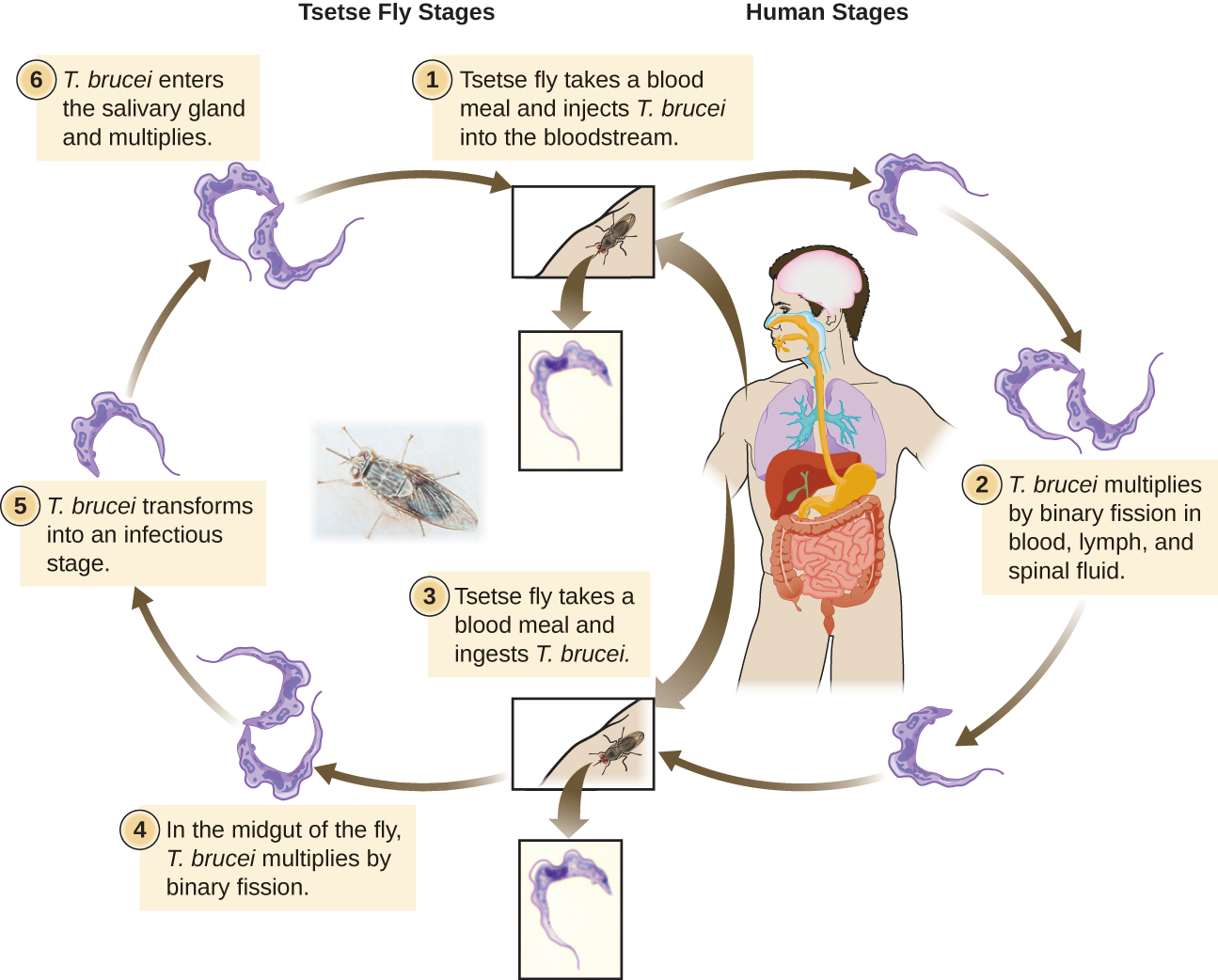
The Euglenozoa also include the trypanosomes, which are parasitic pathogens. The genus Trypanosoma includes T. brucei, which causes African trypanosomiasis (African sleeping sickness and T. cruzi, which causes American trypanosomiasis (Chagas disease). These tropical diseases are spread by insect bites. In African sleeping sickness, T. brucei colonizes the blood and the brain after being transmitted via the bite of a tsetse fly (Glossina spp.) (Figure \(\PageIndex{8}\)). The early symptoms include confusion, difficulty sleeping, and lack of coordination. Left untreated, it is fatal. Chagas’ disease originated and is most common in Latin America. The disease is transmitted by Triatoma spp., insects often called “kissing bugs,” and affects either the heart tissue or tissues of the digestive system. Untreated cases can eventually lead to heart failure or significant digestive or neurological disorders.
The Centers for Disease Control and Prevention (CDC) is responsible for identifying public health priorities in the United States and developing strategies to address areas of concern. As part of this mandate, the CDC has officially identified five parasitic diseases it considers to have been neglected (i.e., not adequately studied). These neglected parasitic infections (NPIs) include toxoplasmosis, Chagas disease, toxocariasis (a nematode infection transmitted primarily by infected dogs), cysticercosis (a disease caused by a tissue infection of the tapeworm Taenia solium), and trichomoniasis (a sexually transmitted disease caused by the parabasalid Trichomonas vaginalis).
The decision to name these specific diseases as NPIs means that the CDC will devote resources toward improving awareness and developing better diagnostic testing and treatment through studies of available data. The CDC may also advise on treatment of these diseases and assist in the distribution of medications that might otherwise be difficult to obtain.3
Of course, the CDC does not have unlimited resources, so by prioritizing these five diseases, it is effectively deprioritizing others. Given that many Americans have never heard of many of these NPIs, it is fair to ask what criteria the CDC used in prioritizing diseases. According to the CDC, the factors considered were the number of people infected, the severity of the illness, and whether the illness can be treated or prevented. Although several of these NPIs may seem to be more common outside the United States, the CDC argues that many cases in the United States likely go undiagnosed and untreated because so little is known about these diseases.4
What criteria should be considered when prioritizing diseases for purposes of funding or research? Are those identified by the CDC reasonable? What other factors could be considered? Should government agencies like the CDC have the same criteria as private pharmaceutical research labs? What are the ethical implications of deprioritizing other potentially neglected parasitic diseases such as leishmaniasis?
Algae
The algae are autotrophic protists that can be unicellular or multicellular. They are important ecologically and environmentally because they are responsible for the production of approximately 70% of the oxygen and organic matter in aquatic environments. Some types of algae, even those that are microscopic, are regularly eaten by humans and other animals. Additionally, algae are the source for agar, agarose, and carrageenan, solidifying agents used in laboratories and in food production. Although algae are typically not pathogenic, some produce toxins. Harmful algal blooms, which occur when algae grow quickly and produce dense populations, can produce high concentrations of toxins that impair liver and nervous-system function in aquatic animals and humans.
Like protozoans, algae often have complex cell structures. For instance, algal cells can have one or more chloroplasts that contain structures called pyrenoids to synthesize and store starch. The chloroplasts themselves differ in their number of membranes, indicative of secondary or rare tertiary endosymbiotic events. Primary chloroplasts have two membranes—one from the original cyanobacteria that the ancestral eukaryotic cell engulfed, and one from the plasma membrane of the engulfing cell. Chloroplasts in some lineages appear to have resulted from secondary endosymbiosis, in which another cell engulfed a green or red algal cell that already had a primary chloroplast within it. The engulfing cell destroyed everything except the chloroplast and possibly the cell membrane of its original cell, leaving three or four membranes around the chloroplast. Different algal groups have different pigments, which are reflected in common names such as red algae, brown algae, and green algae. Algae may also have a variety of life cycles. Reproduction may be asexual by mitosis or sexual using gametes.
Some algae, the seaweeds, are macroscopic and may be confused with plants. Seaweeds can be red, brown, or green, depending on their photosynthetic pigments. Green algae, in particular, share some important similarities with land plants; however, there are also important distinctions. For example, seaweeds do not have true tissues or organs like plants do. Additionally, seaweeds do not have a waxy cuticle to prevent desiccation. Algae can also be confused with cyanobacteria, photosynthetic bacteria that bear a resemblance to algae; however, cyanobacteria are prokaryotes.
Algal Diversity
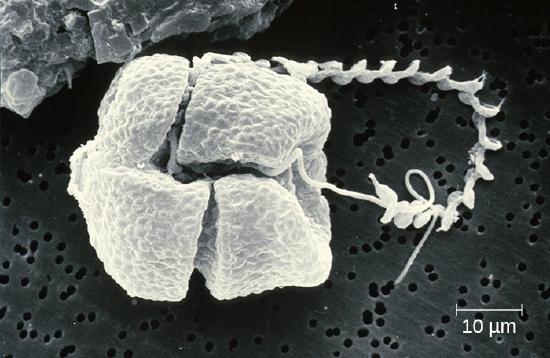
The dinoflagellates are mostly marine organisms and are an important component of plankton. They have a variety of nutritional types and may be phototrophic, heterotrophic, or mixotrophic. Those that are photosynthetic use chlorophyll a, chlorophyll c2, and other photosynthetic pigments (Figure \(\PageIndex{9}\)). They generally have two flagella, causing them to whirl (in fact, the name dinoflagellate comes from the Greek word for “whirl”: dini). Some have cellulose plates forming a hard outer covering, or theca, as armor. Additionally, some dinoflagellates produce neurotoxins that can cause paralysis in humans or fish. Exposure can occur through contact with water containing the dinoflagellate toxins or by feeding on organisms that have eaten dinoflagellates.When a population of dinoflagellates becomes particularly dense, a red tide (a type of harmful algal bloom) can occur. Red tides cause harm to marine life and to humans who consume contaminated marine life. Major toxin producers include the genera Gonyaulax and Alexandrium, both of which cause paralytic shellfish poisoning. Another species, Pfiesteria piscicida, is known as a fish killer because, at certain parts of its life cycle, it can produce toxins harmful to fish and it appears to be responsible for a suite of symptoms, including memory loss and confusion, in humans exposed to water containing the species.

Figure \(\PageIndex{9}\): (a) These large multicellular kelps are members of the brown algae. Note the “leaves” and “stems” that make them appear similar to green plants. (b) This is a species of red algae that is also multicellular. (c) The green alga Halimeda incrassata, shown here growing on the sea floor in shallow water, appears to have plant-like structures, but is not a true plant. (d) Bioluminesence, visible in the cresting wave in this picture, is a phenomenon of certain dinoflagellates. (e) Diatoms (pictured in this micrograph) produce silicaceous tests (skeletons) that form diatomaceous earths. (f) Colonial green algae, like volvox in these three micrographs, exhibit simple cooperative associations of cells. (credit a, e: modification of work by NOAA; credit b: modification of work by Ed Bierman; credit c: modification of work by James St. John; credit d: modification of work by “catalano82”/Flickr; credit f: modification of work by Dr. Ralf Wagner)
Helminths
Parasitic helminths are animals that are often included within the study of microbiology because many species of these worms are identified by their microscopic eggs and larvae. There are two major groups of parasitic helminths: the roundworms (Nematoda) and flatworms (Platyhelminthes). Of the many species that exist in these groups, about half are parasitic and some are important human pathogens. As animals, they are multicellular and have organ systems. However, the parasitic species often have limited digestive tracts, nervous systems, and locomotor abilities. Parasitic forms may have complex reproductive cycles with several different life stages and more than one type of host. Some are monoecious, having both male and female reproductive organs in a single individual, while others are dioecious, each having either male or female reproductive organs.
Nematoda (Roundworms)
Phylum Nematoda (the roundworms) is a diverse group containing more than 15,000 species, of which several are important human parasites (Figure \(\PageIndex{11}\)). These unsegmented worms have a full digestive system even when parasitic. Some are common intestinal parasites, and their eggs can sometimes be identified in feces or around the anus of infected individuals. Ascaris lumbricoides is the largest nematode intestinal parasite found in humans; females may reach lengths greater than 1 meter. A. lumbricoides is also very widespread, even in developed nations, although it is now a relatively uncommon problem in the United States. It may cause symptoms ranging from relatively mild (such as a cough and mild abdominal pain) to severe (such as intestinal blockage and impaired growth).
Trichinellosis, also called trichinosis, caused by Trichinella spiralis, is contracted by consuming undercooked meat, which releases the larvae and allows them to encyst in muscles. Infection can cause fever, muscle pains, and digestive system problems; severe infections can lead to lack of coordination, breathing and heart problems, and even death. Finally, heartworm in dogs and other animals is caused by the nematode Dirofilaria immitis, which is transmitted by mosquitoes. Symptoms include fatigue and cough; when left untreated, death may result.
Of all nematode infections in the United States, pinworm (caused by Enterobius vermicularis) is the most common. Pinworm causes sleeplessness and itching around the anus, where the female worms lay their eggs during the night. Toxocara canis and T. cati are nematodes found in dogs and cats, respectively, that can be transmitted to humans, causing toxocariasis. Antibodies to these parasites have been found in approximately 13.9% of the U.S. population, suggesting that exposure is common.5 Infection can cause larval migrans, which can result in vision loss and eye inflammation, or fever, fatigue, coughing, and abdominal pain, depending on whether the organism infects the eye or the viscera. Another common nematode infection is hookworm, which is caused by Necator americanus (the New World or North American hookworm) and Ancylostoma duodenale (the Old World hookworm). Symptoms of hookworm infection can include abdominal pain, diarrhea, loss of appetite, weight loss, fatigue, and anemia.

The physician explains to Sarah’s mother that ringworm can be transferred between people through touch. “It’s common in school children, because they often come in close contact with each other, but anyone can become infected,” he adds. “Because you can transfer it through objects, locker rooms and public pools are also a potential source of infection. It’s very common among wrestlers and athletes in other contact sports.”
Looking very uncomfortable, Sarah says to her mother “I want this worm out of me.”
The doctor laughs and says, “Sarah, you’re in luck because ringworm is just a name; it is not an actual worm. You have nothing wriggling around under your skin.”
“Then what is it?” asks Sarah.
- What type of pathogen causes ringworm?
- What is the most common nematode infection in the United States?
Platyhelminths (Flatworms)
Phylum Platyhelminthes (the platyhelminths) are flatworms. This group includes the flukes, tapeworms, and the turbellarians, which include planarians. The flukes and tapeworms are medically important parasites (Figure \(\PageIndex{12}\)).
The flukes (trematodes) are nonsegmented flatworms that have an oral sucker (Figure \(\PageIndex{13}\)) (and sometimes a second ventral sucker) and attach to the inner walls of intestines, lungs, large blood vessels, or the liver. Trematodes have complex life cycles, often with multiple hosts. Several important examples are the liver flukes (Clonorchis and Opisthorchis), the intestinal fluke (Fasciolopsis buski), and the oriental lung fluke (Paragonimus westermani). Schistosomiasis is a serious parasitic disease, considered second in the scale of its impact on human populations only to malaria. The parasites Schistosoma mansoni, S. haematobium, and S. japonicum, which are found in freshwater snails, are responsible for schistosomiasis. Immature forms burrow through the skin into the blood. They migrate to the lungs, then to the liver and, later, other organs. Symptoms include anemia, malnutrition, fever, abdominal pain, fluid buildup, and sometimes death.
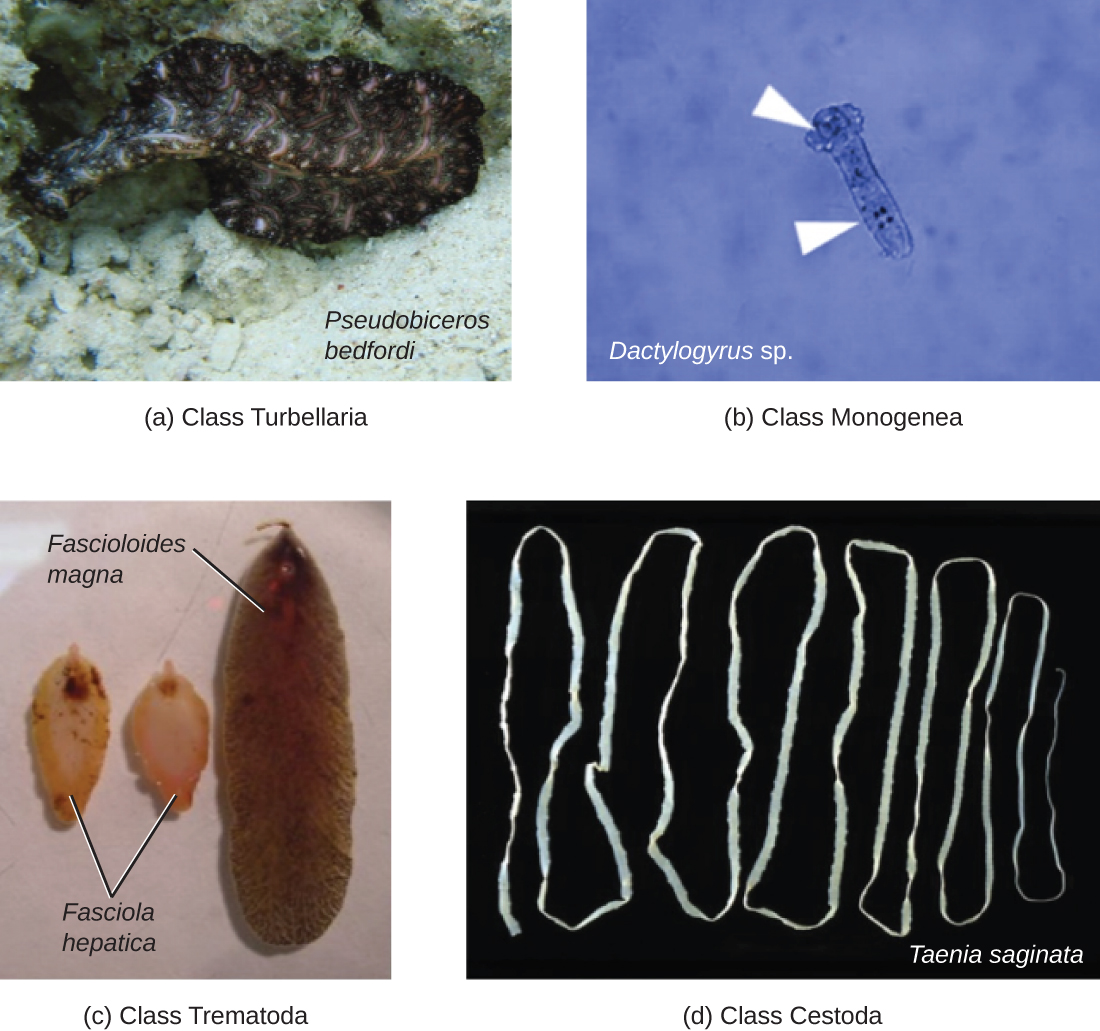
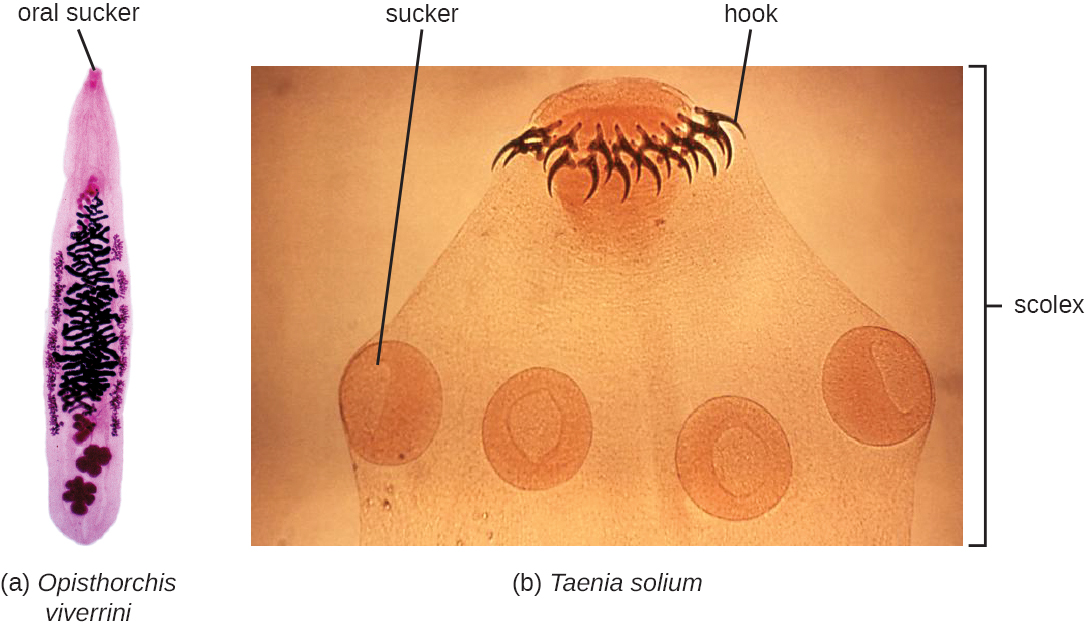
The other medically important group of platyhelminths are commonly known as tapeworms (cestodes) and are segmented flatworms that may have suckers or hooks at the scolex (head region) (Figure \(\PageIndex{13}\)). Tapeworms use these suckers or hooks to attach to the wall of the small intestine. The body of the worm is made up of segments called proglottids that contain reproductive structures; these detach when the gametes are fertilized, releasing gravid proglottids with eggs. Tapeworms often have an intermediate host that consumes the eggs, which then hatch into a larval form called an oncosphere. The oncosphere migrates to a particular tissue or organ in the intermediate host, where it forms cysticerci. After being eaten by the definitive host, the cysticerci develop into adult tapeworms in the host's digestive system. Taenia saginata (the beef tapeworm) and T. solium (the pork tapeworm) enter humans through ingestion of undercooked, contaminated meat. The adult worms develop and reside in the intestine, but the larval stage may migrate and be found in other body locations such as skeletal and smooth muscle. The beef tapeworm is relatively benign, although it can cause digestive problems and, occasionally, allergic reactions. The pork tapeworm can cause more serious problems when the larvae leave the intestine and colonize other tissues, including those of the central nervous system. Diphylobothrium latum is the largest human tapeworm and can be ingested in undercooked fish. It can grow to a length of 15 meters. Echinococcus granulosus, the dog tapeworm, can parasitize humans and uses dogs as an important host.
For residents of temperate, developed countries, it may be difficult to imagine just how common helminth infections are in the human population. In fact, they are quite common and even occur frequently in the United States. Worldwide, approximately 807–1,221 million people are infected with Ascaris lumbricoides (perhaps one-sixth of the human population) and far more are infected if all nematode species are considered.6 Rates of infection are relatively high even in industrialized nations. Approximately 604–795 million people are infected with whipworm (Trichuris) worldwide (Trichuris can also infect dogs), and 576–740 million people are infected with hookworm (Necator americanus and Ancylostoma duodenale).7 Toxocara, a nematode parasite of dogs and cats, is also able to infect humans. It is widespread in the United States, with about 10,000 symptomatic cases annually. However, one study found 14% of the population (more than 40 million Americans) was seropositive, meaning they had been exposed to the parasite at one time. More than 200 million people have schistosomiasis worldwide. Most of the World Health Organization (WHO) neglected tropical diseases are helminths. In some cases, helminths may cause subclinical illnesses, meaning the symptoms are so mild that that they go unnoticed. In other cases, the effects may be more severe or chronic, leading to fluid accumulation and organ damage. With so many people affected, these parasites constitute a major global public health concern.
Dracunculiasis, or Guinea worm disease, is caused by a nematode called Dracunculus medinensis. When people consume contaminated water, water fleas (small crustaceans) containing the nematode larvae may be ingested. These larvae migrate out of the intestine, mate, and move through the body until females eventually emerge (generally through the feet or lower limbs). While Guinea worm disease is rarely fatal, it is extremely painful and can be accompanied by secondary infections and edema (Figure \(\PageIndex{14}\)).
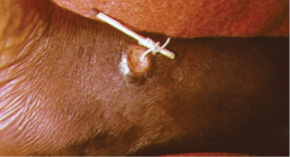
An eradication campaign led by WHO, the CDC, the United Nations Children’s Fund (UNICEF), and the Carter Center (founded by former U.S. president Jimmy Carter) has been extremely successful in reducing cases of dracunculiasis. This has been possible because diagnosis is straightforward, there is an inexpensive method of control, there is no animal reservoir, the water fleas are not airborne (they are restricted to still water), the disease is geographically limited, and there has been a commitment from the governments involved. Additionally, no vaccines or medication are required for treatment and prevention. In 1986, 3.5 million people were estimated to be affected. After the eradication campaign, which included helping people in affected areas learn to filter water with cloth, only four countries continue to report the disease (Chad, Mali, South Sudan, and Ethiopia) with a total of 126 cases reported to WHO in 2014.
Fungi
The fungi comprise a diverse group of organisms that are heterotrophic and typically saprozoic. In addition to the well-known macroscopic fungi (such as mushrooms and molds), many unicellular yeasts and spores of macroscopic fungi are microscopic. For this reason, fungi are included within the field of microbiology.
Fungi are important to humans in a variety of ways. Both microscopic and macroscopic fungi have medical relevance, with some pathogenic species that can cause mycoses (illnesses caused by fungi). Some pathogenic fungi are opportunistic, meaning that they mainly cause infections when the host’s immune defenses are compromised and do not normally cause illness in healthy individuals. Fungi are important in other ways. They act as decomposers in the environment, and they are critical for the production of certain foods such as cheeses. Fungi are also major sources of antibiotics, such as penicillin from the fungus Penicillium.
There are notable unique features in fungal cell walls and membranes. Fungal cell walls contain chitin, as opposed to the cellulose found in the cell walls of plants and many protists. Additionally, whereas animals have cholesterol in their cell membranes, fungal cell membranes have different sterols called ergosterols. Ergosterols are often exploited as targets for antifungal drugs.
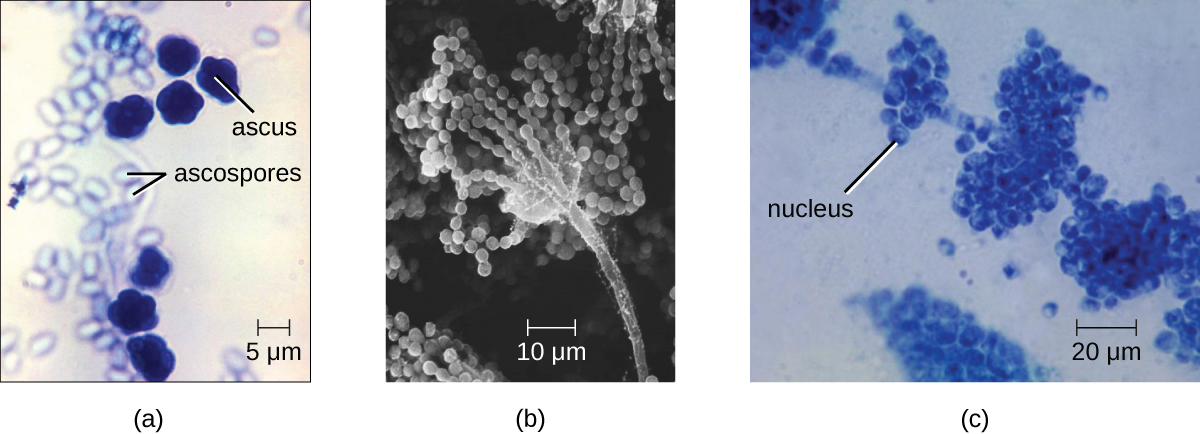
Fungal life cycles are unique and complex. Fungi reproduce sexually either through cross- or self-fertilization. Haploid fungi can form hyphae that have gametes at the tips. Two different mating types (represented as “+ type” and “– type”) are involved. The cytoplasms of the + and – type gametes fuse (in an event called plasmogamy), producing a cell with two distinct nuclei (a dikaryotic cell). Later, the nuclei fuse (in an event called karyogamy) to create a diploid zygote. The zygote undergoes meiosis to form spores that germinate to start the haploid stage, which eventually creates more haploid mycelia. Depending on the taxonomic group, these sexually produced spores are known as zygospores (in Zygomycota), ascospores (in Ascomycota), or basidiospores (in Basidiomycota).
Fungi may also exhibit asexual reproduction by mitosis, mitosis with budding, fragmentation of hyphae, and formation of asexual spores by mitosis. These spores are specialized cells that, depending on the organism, may have unique characteristics for survival, reproduction, and dispersal. Fungi exhibit several types of asexual spores and these can be important in classification.
Molds
Fungi have well-defined characteristics that set them apart from other organisms. Most multicellular fungal bodies, commonly called molds, are made up of filaments called hyphae. Hyphae can form a tangled network called a mycelium and form the thallus (body) of fleshy fungi. Hyphae that have walls between the cells are called septate hyphae; hyphae that lack walls and cell membranes between the cells are called nonseptate or coenocytic hyphae). (Figure \(\PageIndex{15}\)). Macroscopically these create a velvety appearance as the colony grows.
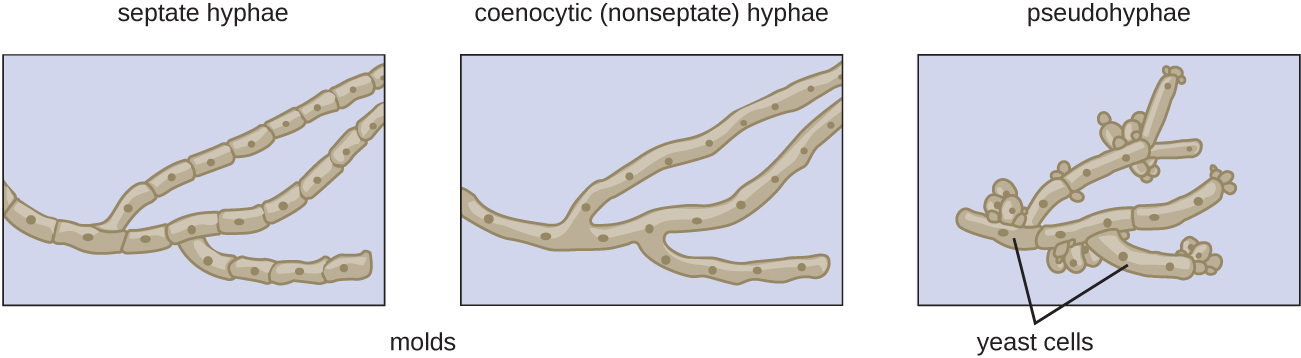
 Figure \(\PageIndex{16}\): These images show asexually produced spores. (a) This brightfield micrograph shows the release of spores from a sporangium at the end of a hypha called a sporangiophore. The organism is a Mucor sp. fungus, a mold often found indoors. (b) Sporangia grow at the ends of stalks, which appear as the white fuzz seen on this bread mold, Rhizopus stolonifer. The tips of bread mold are the dark, spore-containing sporangia. (credit a: modification of work by Centers for Disease Control and Prevention; credit b right: modification of work by “Andrew”/Flickr)
Figure \(\PageIndex{16}\): These images show asexually produced spores. (a) This brightfield micrograph shows the release of spores from a sporangium at the end of a hypha called a sporangiophore. The organism is a Mucor sp. fungus, a mold often found indoors. (b) Sporangia grow at the ends of stalks, which appear as the white fuzz seen on this bread mold, Rhizopus stolonifer. The tips of bread mold are the dark, spore-containing sporangia. (credit a: modification of work by Centers for Disease Control and Prevention; credit b right: modification of work by “Andrew”/Flickr)
Yeasts
In contrast to molds, yeasts are unicellular fungi. The budding yeasts reproduce asexually by budding off a smaller daughter cell; the resulting cells may sometimes stick together as a short chain or pseudohypha (Figure \(\PageIndex{15}\)). Candida albicans is a common yeast that forms pseudohyphae; it is associated with various infections in humans, including vaginal yeast infections, oral thrush, and candidiasis of the skin.
Saccharomyces yeasts, including the baker’s yeast S. cerevisiae, are unicellular ascomycetes with haploid and diploid stages (Figure \(\PageIndex{17}\)). This and other Saccharomyces species are used for brewing beer. Cryptococcus neoformans, a fungus commonly found as a yeast in the environment, can cause serious lung infections when inhaled by individuals with weakened immune systems.
Dimorphic
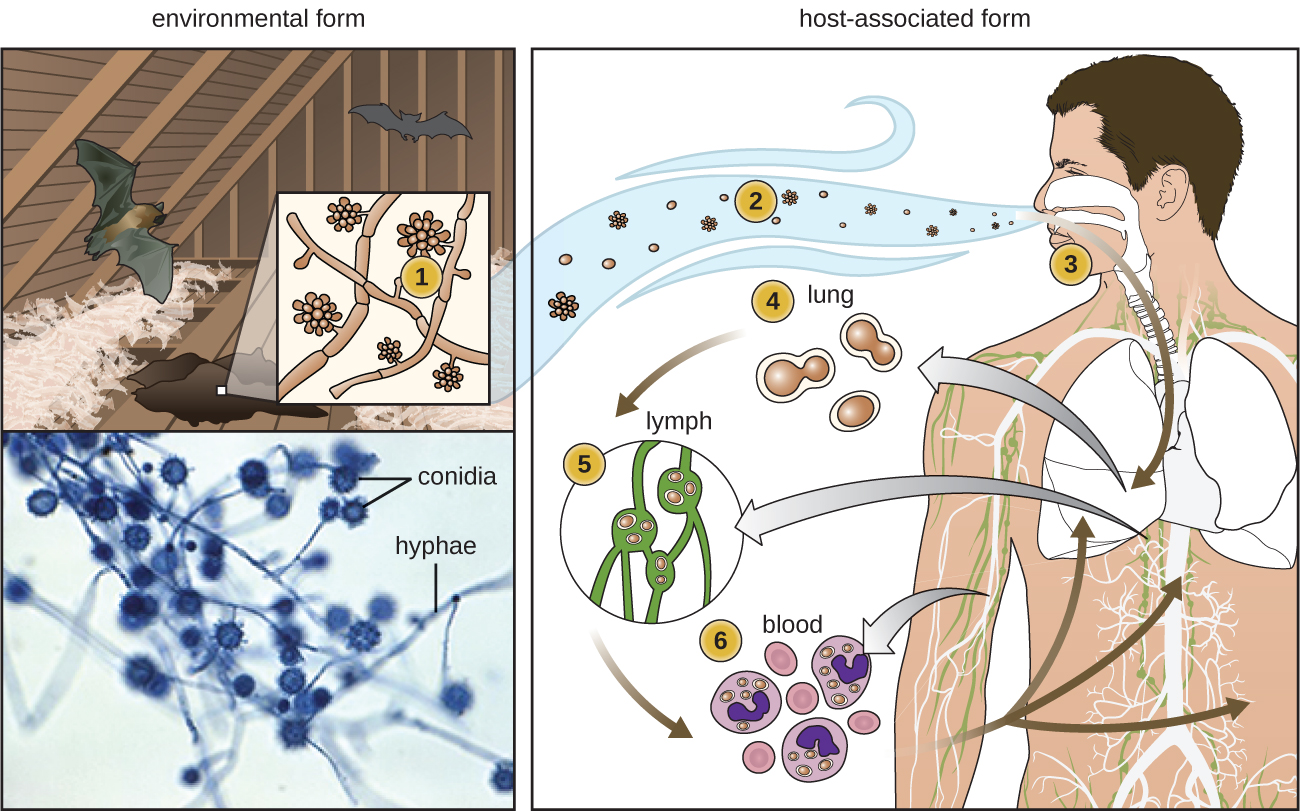
Some fungi are dimorphic, having more than one appearance during their life cycle. These dimorphic fungi may be able to appear as yeasts or molds, which can be important for infectivity but a pain when trying to determine the organism responsible for disease. They are capable of changing their appearance in response to environmental changes such as nutrient availability or fluctuations in temperature, growing as a mold, for example, at 25 °C (77 °F), and as yeast cells at 37 °C (98.6 °F). This ability helps dimorphic fungi to survive in diverse environments. Histoplasma capsulatum, the pathogen that causes histoplasmosis, a lung infection, is an example of a dimorphic fungus (Figure \(\PageIndex{18}\)).
When we think about antimicrobial medications, antibiotics such as penicillin often come to mind. Penicillin and related antibiotics interfere with the synthesis of peptidoglycan cell walls, which effectively targets bacterial cells. These antibiotics are useful because humans (like all eukaryotes) do not have peptidoglycan cell walls.
Developing medications that are effective against eukaryotic cells but not harmful to human cells is more difficult. Despite huge morphological differences, the cells of humans, fungi, helminths and protists are similar in terms of their ribosomes, cytoskeletons, and cell membranes. As a result, it is more challenging to develop medications that target protozoans and fungi in the same way that antibiotics target prokaryotes.
Fungicides have relatively limited modes of action. Because fungi have ergosterols (instead of cholesterol) in their cell membranes, the different enzymes involved in sterol production can be a target of some medications. The azole and morpholine fungicides interfere with the synthesis of membrane sterols. These are used widely in agriculture (fenpropimorph) and clinically (e.g., miconazole). Some antifungal medications target the chitin cell walls of fungi. Despite the success of these compounds in targeting fungi, antifungal medications for systemic infections still tend to have more toxic side effects than antibiotics for bacteria.
Sarah is relieved the ringworm is not an actual worm, but wants to know what it really is. The physician explains that ringworm is a fungus. He tells her that she will not see mushrooms popping out of her skin, because this fungus is more like the invisible part of a mushroom that hides in the soil. He reassures her that they are going to get the fungus out of her.
The doctor cleans and then carefully scrapes the lesion to place a specimen on a slide. By looking at it under a microscope, the physician is able to confirm that a fungal infection is responsible for Sarah’s lesion. Even if the pathogen resembled a helminth under the microscope, the presence of cell walls would rule out the possibility because animal cells lack cell walls. The doctor prescribes an antifungal cream for Sarah’s mother to apply to the ringworm. Sarah’s mother asks, “What should we do if it doesn’t go away?”
The doctor explains that ringworm is a general term for a condition caused by multiple species. The first step is to take a scraping for examination under the microscope, which the doctor has already done. He explains that he has identified the infection as a fungus, and that the antifungal cream works against the most common fungi associated with ringworm. However, the cream may not work against some species of fungus.
If the cream is not working after a couple of weeks, Sarah should come in for another visit, at which time the doctor will take steps to identify the species of the fungus.
Positive identification of dermatophytes requires culturing. For this purpose, Sabouraud’s agar may be used. In the case of Sarah’s infection, which cleared up within 2 weeks of treatment, the culture would have a granular texture and would appear pale pink on top and red underneath. These features suggest that the fungus is Trichophyton rubrum, a common cause of ringworm.
Key Concepts and Summary
- Protists are a diverse, polyphyletic group of eukaryotic organisms.
- Protists may be unicellular or multicellular. They vary in how they get their nutrition, morphology, method of locomotion, and mode of reproduction.
- The protozoa group include important pathogens and parasites.
- Algae are a diverse group of photosynthetic eukaryotic protists
- Algae may be unicellular or multicellular. Large, multicellular algae are called seaweeds but are not plants and lack plant-like tissues and organs
- Helminth parasites are included within the study of microbiology because they are often identified by looking for microscopic eggs and larvae.
- The two major groups of helminth parasites are the roundworms (Nematoda) and the flatworms (Platyhelminthes).
- The fungi include diverse saprotrophic eukaryotic organisms with chitin cell walls
- Fungi can be unicellular or multicellular; some (like yeast) and fungal spores are microscopic, whereas some are large and conspicuous.
- Important differences in fungal cells, such as ergosterols in fungal membranes, can be targets for antifungal medications, but similarities between human and fungal cells make it difficult to find targets for medications and these medications often have toxic adverse effects
Footnotes
- J. Flegr et al. “Toxoplasmosis—A Global Threat. Correlation of Latent Toxoplasmosis With Specific Disease Burden in a Set of 88 Countries.” PloS ONE 9 no. 3 (2014):e90203.
- J. Flegr. “Effects of Toxoplasma on Human Behavior.” Schizophrenia Bull 33, no. 3 (2007):757–760.
- Centers for Disease Control and Prevention. “Neglected Parasitic Infections (NPIs) in the United States.” http://www.cdc.gov/parasites/npi/. Last updated July 10, 2014.
- Centers for Disease Control and Prevention. “Fact Sheet: Neglected Parasitic Infections in the United States.” www.cdc.gov/parasites/resourc..._factsheet.pdf
- Won K, Kruszon-Moran D, Schantz P, Jones J. “National seroprevalence and risk factors for zoonotic Toxocara spp. infection.” In: Abstracts of the 56th American Society of Tropical Medicine and Hygiene; Philadelphia, Pennsylvania; 2007 Nov 4-8.
- Fenwick, A. “The global burden of neglected tropical diseases.” Public health 126 no.3 (Mar 2012): 233–6.
- de Silva, N., et. al. (2003). “Soil-transmitted helminth infections: updating the global picture”. Trends in Parasitology 19 (December 2003): 547–51.
- World Health Organization. “South Sudan Reports Zero Cases of Guinea-Worm Disease for Seventh Consecutive Month.” 2016. http://www.who.int/dracunculiasis/no...ive_months/en/. Accessed May 2, 2016.
Contributors and Attributions
Nina Parker, (Shenandoah University), Mark Schneegurt (Wichita State University), Anh-Hue Thi Tu (Georgia Southwestern State University), Philip Lister (Central New Mexico Community College), and Brian M. Forster (Saint Joseph’s University) with many contributing authors. Original content via Openstax (CC BY 4.0; Access for free at https://openstax.org/books/microbiology/pages/1-introduction)


