2.7: Nucleic Acids
- Page ID
- 75825
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Describe the structure of nucleic acids and define the types of nucleic acids
- Explain the structure and role of DNA
- Explain the structure and roles of RNA
- Explain the structure and role of ATP
Nucleic acids are the most important macromolecules for the continuity of life. They carry the genetic blueprint of a cell and carry instructions for the functioning of the cell.
DNA and RNA
The two main types of nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). DNA is the genetic material found in all living organisms, ranging from single-celled bacteria to multicellular mammals. It is found in the nucleus of eukaryotes and in the organelles, chloroplasts, and mitochondria. In prokaryotes, the DNA is not enclosed in a membranous envelope.
The entire genetic content of a cell is known as its genome, and the study of genomes is genomics. This includes both the chromosomal and extra-chromosomal DNA. Chromosomes in prokaryotes have very few differences when compared to eukaryotes. These differences are mostly in size, number and shape. Both have packaging proteins, both contain many genes. Eukaryotes generally have more DNA, higher numbers and a linear shape, as compared to circular in prokaryotes. Also in eukaryotic cells, DNA forms a complex with histone proteins to form chromatin, the substance of eukaryotic chromosomes. A chromosome may contain hundreds to tens of thousands of genes. Many genes contain the information to make protein products; other genes code for RNA products. DNA controls all of the cellular activities by turning the genes “on” or “off.”
Another type of nucleic acid, RNA, is mostly involved in protein synthesis. The DNA molecules never leave the nucleus (in eukaryotes) or nucleoid (in prokaryotes) but instead use an intermediary to communicate with the rest of the cell. This intermediary is the messenger RNA (mRNA). Other types of RNA—like rRNA, tRNA, and microRNA—are also involved in protein synthesis and its regulation.
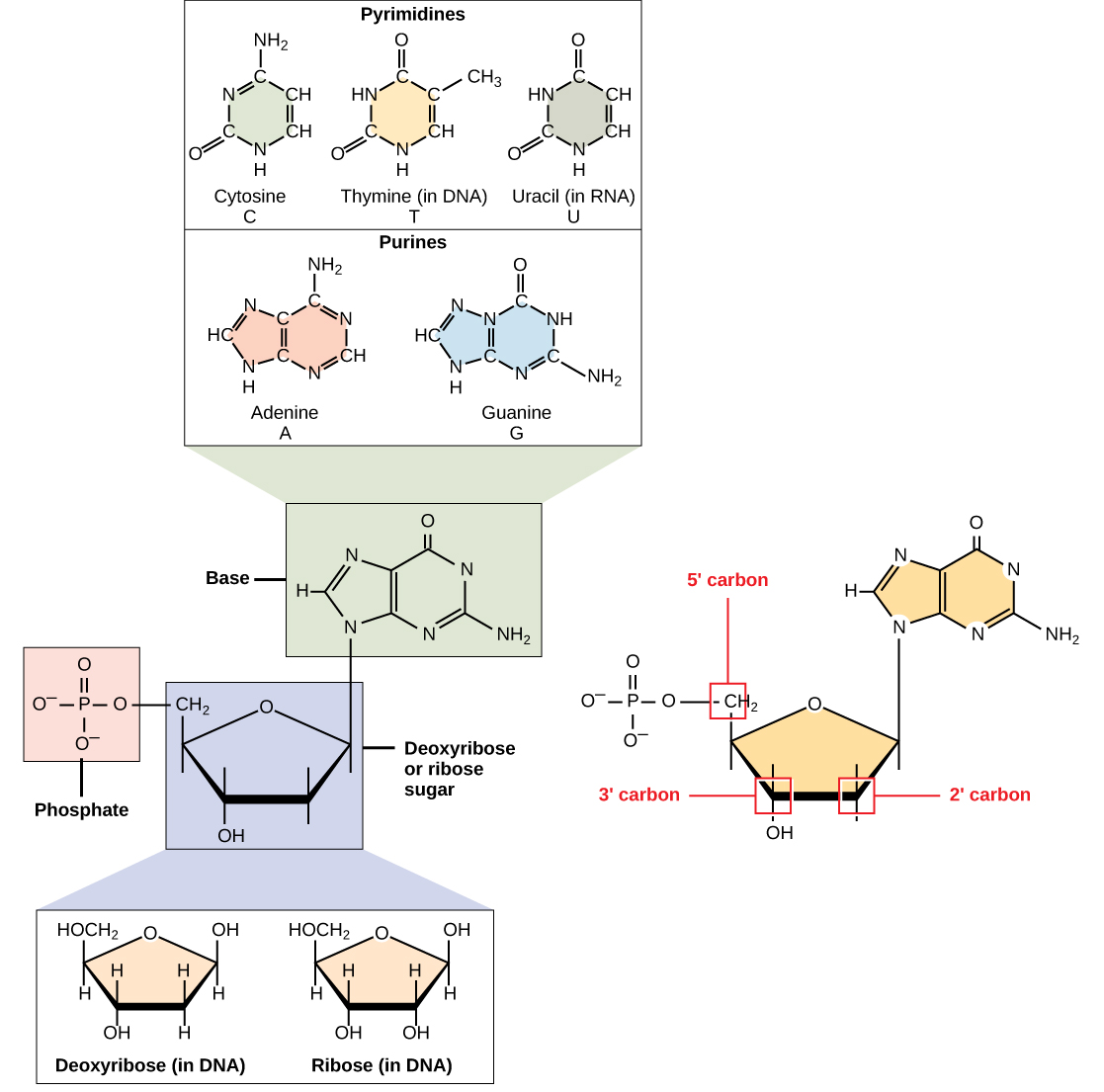
DNA and RNA are polymers made up of monomers known as nucleotides. The nucleotides combine can with each other to form a polynucleotide. Each nucleotide is made up of three components: a nitrogenous base, a pentose (five-carbon) sugar, and one or more phosphate groups (Figure \(\PageIndex{1}\)). Each nitrogenous base in a nucleotide is attached to a sugar molecule, which is attached to the phosphate group(s).
The nitrogenous bases, important components of nucleotides, are organic molecules and are so named because they contain carbon and nitrogen. They are bases because they contain an amino group that has the potential of binding an extra hydrogen, and thus, decreases the hydrogen ion concentration in its environment, making it more basic. Each nucleotide in DNA contains one of four possible nitrogenous bases: adenine (A), guanine (G) cytosine (C), and thymine (T).
Adenine and guanine are classified as purines. The primary structure of a purine is two carbon-nitrogen rings. Cytosine, thymine, and uracil are classified as pyrimidines which have a single carbon-nitrogen ring as their primary structure (Figure \(\PageIndex{1}\)). Each of these basic carbon-nitrogen rings has different functional groups attached to it. In molecular biology shorthand, the nitrogenous bases are simply known by their symbols A, T, G, C, and U. DNA contains A, T, G, and C whereas RNA contains A, U, G, and C.
The pentose sugar in DNA is deoxyribose, and in RNA, the sugar is ribose (Figure \(\PageIndex{1}\)). The difference between the sugars is the presence of the hydroxyl group on the second carbon of the ribose and hydrogen on the second carbon of the deoxyribose. The carbon atoms of the sugar molecule are numbered as 1′, 2′, 3′, 4′, and 5′ (1′ is read as “one prime”). The phosphate residue is attached to the hydroxyl group of the 5′ carbon of one sugar and the hydroxyl group of the 3′ carbon of the sugar of the next nucleotide, which forms a 5′–3′ phosphodiester linkage. The phosphodiester linkage is not formed by simple dehydration reaction like the other linkages connecting monomers in macromolecules: its formation involves the removal of two phosphate groups. A polynucleotide may have thousands of such phosphodiester linkages.
DNA Double-Helix Structure
DNA has a double-helix structure (Figure \(\PageIndex{2}\)). The sugar and phosphate lie on the outside of the helix, forming the backbone of the DNA. The nitrogenous bases are stacked in the interior, like the steps of a staircase, in pairs; the pairs are bound to each other by hydrogen bonds. Every base pair in the double helivx is separated from the next base pair by 0.34 nm. The two strands of the helix run in opposite directions, meaning that the 5′ carbon end of one strand will face the 3′ carbon end of its matching strand. (This is referred to as antiparallel orientation and is important to DNA replication and in many nucleic acid interactions.)
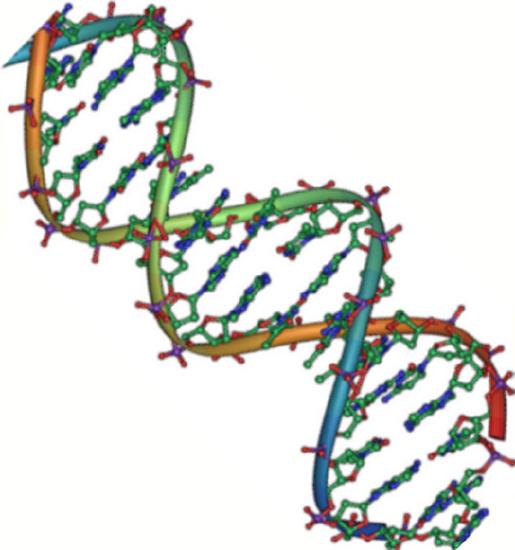
Only certain types of base pairing are allowed. For example, a certain purine can only pair with a certain pyrimidine. This means A can pair with T, and G can pair with C, as shown in Figure \(\PageIndex{3}\). This is known as the base complementary rule. In other words, the DNA strands are complementary to each other. If the sequence of one strand is AATTGGCC, the complementary strand would have the sequence TTAACCGG. During DNA replication, each strand is copied, resulting in a daughter DNA double helix containing one parental DNA strand and a newly synthesized strand.
RNA
Ribonucleic acid, or RNA, is mainly involved in the process of protein synthesis under the direction of DNA. RNA is usually single-stranded and is made of ribonucleotides that are linked by phosphodiester bonds. A ribonucleotide in the RNA chain contains ribose (the pentose sugar), one of the four nitrogenous bases (A, U, G, and C), and the phosphate group. Even though the RNA is single stranded, most RNA types show extensive intramolecular base pairing between complementary sequences, creating a predictable three-dimensional structure essential for their function.
There are four major types of RNA: messenger RNA (mRNA), ribosomal RNA (rRNA), transfer RNA (tRNA), and microRNA (miRNA). The first, mRNA, carries the message from DNA, which controls all of the cellular activities in a cell. If a cell requires a certain protein to be synthesized, the gene for this product is turned “on” and the messenger RNA is synthesized in the nucleus. The RNA base sequence is complementary to the coding sequence of the DNA from which it has been copied. However, in RNA, the base T is absent and U is present instead. If the DNA strand has a sequence AATTGCGC, the sequence of the complementary RNA is UUAACGCG. In the cytoplasm, the mRNA interacts with ribosomes and other cellular machinery (Figure \(\PageIndex{3}\)). More is described about their roles in later chapters.
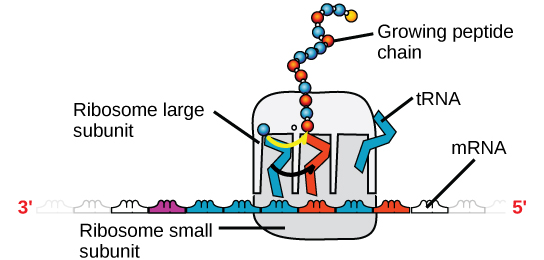
Table \(\PageIndex{1}\) below summarizes features of DNA and RNA.
Table \(\PageIndex{1}\): Features of DNA and RNA.
| Features of DNA and RNA | ||
|---|---|---|
| DNA | RNA | |
| Function | Carries genetic information | Involved in protein synthesis |
| Location | Remains in the nucleus | Leaves the nucleus |
| Structure | Double helix | Usually single-stranded |
| Sugar | Deoxyribose | Ribose |
| Pyrimidines | Cytosine, thymine | Cytosine, uracil |
| Purines | Adenine, guanine | Adenine, guanine |
As you have learned, information flow in an organism takes place from DNA to RNA to protein. DNA dictates the structure of mRNA in a process known as transcription, and RNA dictates the structure of protein in a process known as translation. This is known as the Central Dogma of Life, which holds true for all organisms; however, exceptions to the rule occur in connection with viral infections.
 To learn more about DNA, explore the Howard Hughes Medical Institute BioInteractive animations on the topic of DNA.
To learn more about DNA, explore the Howard Hughes Medical Institute BioInteractive animations on the topic of DNA.
ATP
ATP is a small, relatively simple molecule (Figure \(\PageIndex{4}\)), but within some of its bonds, it contains the potential for a quick burst of energy that can be harnessed to perform cellular work. This molecule can be thought of as the primary energy currency of cells in much the same way that money is the currency that people exchange for things they need. ATP is used to power the majority of energy-requiring cellular reactions.

As its name suggests, adenosine triphosphate is comprised of adenosine bound to three phosphate groups (Figure \(\PageIndex{5}\)). Adenosine is a nucleotide consisting of the nitrogenous base adenine and a five-carbon sugar, ribose. The three phosphate groups, in order of closest to furthest from the ribose sugar, are labeled alpha, beta, and gamma. Together, these chemical groups constitute an energy powerhouse. However, not all bonds within this molecule exist in a particularly high-energy state. Both bonds that link the phosphates are equally high-energy bonds (phosphoanhydride bonds) that, when broken, release sufficient energy to power a variety of cellular reactions and processes. These high-energy bonds are the bonds between the second and third (or beta and gamma) phosphate groups and between the first and second phosphate groups. The reason that these bonds are considered “high-energy” is because the products of such bond breaking—adenosine diphosphate (ADP) and one inorganic phosphate group (Pi)—have considerably lower free energy than the reactants: ATP and a water molecule. Because this reaction takes place with the use of a water molecule, it is considered a hydrolysis reaction.
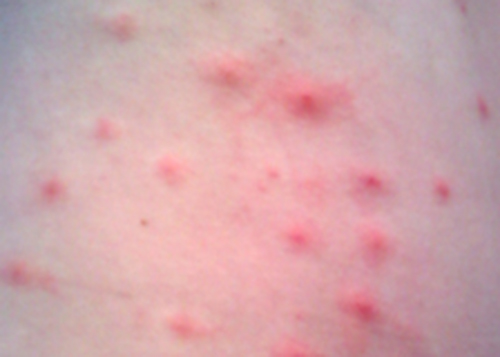
Penny stopped using her new sunscreen and applied the corticosteroid cream to her rash as directed. However, after several days, her rash had not improved and actually seemed to be getting worse. She made a follow-up appointment with her doctor, who observed a bumpy red rash and pus-filled blisters around hair follicles (Figure \(\PageIndex{3}\)). The rash was especially concentrated in areas that would have been covered by a swimsuit. After some questioning, Penny told the physician that she had recently attended a pool party and spent some time in a hot tub. In light of this new information, the doctor suspected a case of hot tub rash, an infection frequently caused by the bacterium Pseudomonas aeruginosa, an opportunistic pathogen that can thrive in hot tubs and swimming pools, especially when the water is not sufficiently chlorinated. P. aeruginosa is the same bacterium that is associated with infections in the lungs of patients with cystic fibrosis.
The doctor collected a specimen from Penny’s rash to be sent to the clinical microbiology lab. Confirmatory tests were carried out to distinguish P. aeruginosa from enteric pathogens that can also be present in pool and hot-tub water. The test included the production of the blue-green pigment pyocyanin on cetrimide agar and growth at 42 °C. Cetrimide is a selective agent that inhibits the growth of other species of microbial flora and also enhances the production of P. aeruginosa pigments pyocyanin and fluorescein, which are a characteristic blue-green and yellow-green, respectively.
Tests confirmed the presence of P. aeruginosa in Penny’s skin sample, but the doctor decided not to prescribe an antibiotic. Even though P. aeruginosa is a bacterium, Pseudomonas species are generally resistant to many antibiotics. Luckily, skin infections like Penny’s are usually self-limiting; the rash typically lasts about 2 weeks and resolves on its own, with or without medical treatment. The doctor advised Penny to wait it out and keep using the corticosteroid cream. The cream will not kill the P. aeruginosa on Penny’s skin, but it should calm her rash and minimize the itching by suppressing her body’s inflammatory response to the bacteria.
Key Concepts and Summary
- Nucleic acids are molecules made up of nucleotides that direct cellular activities such as cell division and protein synthesis.
- Each nucleotide is made up of a pentose sugar, a nitrogenous base, and a phosphate group.
- DNA carries the genetic blueprint of the cell and is passed on from parents to offspring (in the form of chromosomes). It has a double-helical structure with the two strands running in opposite directions, connected by hydrogen bonds, and complementary to each other.
- RNA is single-stranded and is made of a pentose sugar (ribose), a nitrogenous base, and a phosphate group. RNA is involved in protein synthesis and its regulation.
- ATP is a single nucleotide made of a pentose sugar (ribose), a nitrogenous base, and three phosphate groups. It helps the cell to store and move energy.
Glossary
- adenosine triphosphate (ATP)
- adenosine triphosphate, the cell’s energy currency
- deoxyribonucleic acid (DNA)
- double-helical molecule that carries the hereditary information of the cell
- messenger RNA (mRNA)
- RNA that carries information from DNA to ribosomes during protein synthesis
- nucleic acid
- biological macromolecule that carries the genetic blueprint of a cell and carries instructions for the functioning of the cell
- nucleotide
- monomer of nucleic acids; contains a pentose sugar, one or more phosphate groups, and a nitrogenous base
- phosphodiester
- linkage covalent chemical bond that holds together the polynucleotide chains with a phosphate group linking two pentose sugars of neighboring nucleotides
- polynucleotide
- long chain of nucleotides
- purine
- type of nitrogenous base in DNA and RNA; adenine and guanine are purines
- pyrimidine
- type of nitrogenous base in DNA and RNA; cytosine, thymine, and uracil are pyrimidines
- ribonucleic acid (RNA)
- single-stranded, often internally base paired, molecule that is involved in protein synthesis
- ribosomal RNA (rRNA)
- RNA that ensures the proper alignment of the mRNA and the ribosomes during protein synthesis and catalyzes the formation of the peptide linkage
- transcription
- process through which messenger RNA forms on a template of DNA
- transfer RNA (tRNA)
- RNA that carries activated amino acids to the site of protein synthesis on the ribosome
- translation
- process through which RNA directs the formation of protein
Contributors and Attributions
Connie Rye (East Mississippi Community College), Robert Wise (University of Wisconsin, Oshkosh), Vladimir Jurukovski (Suffolk County Community College), Jean DeSaix (University of North Carolina at Chapel Hill), Jung Choi (Georgia Institute of Technology), Yael Avissar (Rhode Island College) among other contributing authors. Original content by OpenStax (CC BY 4.0; Download for free at http://cnx.org/contents/185cbf87-c72...f21b5eabd@9.87).


