12.5: The Language of Epidemiologists
- Page ID
- 31871
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Explain the difference between prevalence and incidence of disease
- Distinguish the characteristics of sporadic, endemic, epidemic, and pandemic diseases
- Explain the use of Koch’s postulates and their modifications to determine the etiology of disease
- Summarize Koch’s postulates and molecular Koch’s postulates, respectively, and explain their significance and limitations
- Explain the relationship between epidemiology and public health
- Describe the entities involved in international public health and their activities
- Identify and differentiate between emerging and reemerging infectious diseases
The field of epidemiology concerns the geographical distribution and timing of infectious disease occurrences and how they are transmitted and maintained in nature, with the goal of recognizing and controlling outbreaks. The science of epidemiology includes etiology (the study of the causes of disease) and investigation of disease transmission (mechanisms by which a disease is spread).
Analyzing Disease in a Population
Epidemiological analyses are always carried out with reference to a population, which is the group of individuals that are at risk for the disease or condition. The population can be defined geographically, but if only a portion of the individuals in that area are susceptible, additional criteria may be required. Susceptible individuals may be defined by particular behaviors, such as intravenous drug use, owning particular pets, or membership in an institution, such as a college. Being able to define the population is important because most measures of interest in epidemiology are made with reference to the size of the population.
The state of being diseased is called morbidity. Morbidity in a population can be expressed in a few different ways. Morbidity or total morbidity is expressed in numbers of individuals without reference to the size of the population. The morbidity rate can be expressed as the number of diseased individuals out of a standard number of individuals in the population, such as 100,000, or as a percent of the population.
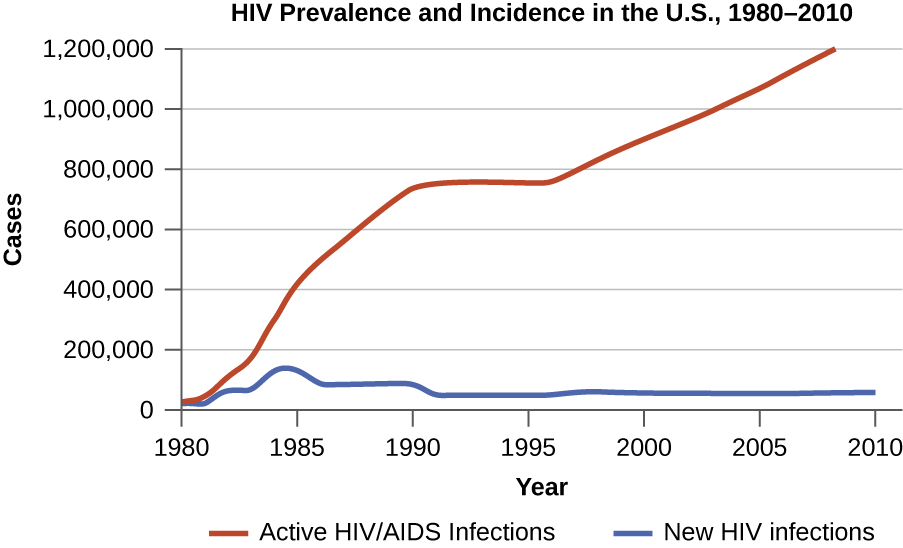
There are two aspects of morbidity that are relevant to an epidemiologist: a disease’s prevalence and its incidence. Prevalence is the number, or proportion, of individuals with a particular illness in a given population at a point in time. For example, the Centers for Disease Control and Prevention (CDC) estimated that in 2012, there were about 1.2 million people 13 years and older with an active human immunodeficiency virus (HIV) infection. Expressed as a proportion, or rate, this is a prevalence of 467 infected persons per 100,000 in the population.1 On the other hand, incidence is the number or proportion of new cases in a period of time. For the same year and population, the CDC estimates that there were 43,165 newly diagnosed cases of HIV infection, which is an incidence of 13.7 new cases per 100,000 in the population.2 The relationship between incidence and prevalence can be seen in Figure \(\PageIndex{1}\). For a chronic disease like HIV infection, prevalence will generally be higher than incidence because it represents the cumulative number of new cases over many years minus the number of cases that are no longer active (e.g., because the patient died or was cured).
In addition to morbidity rates, the incidence and prevalence of mortality (death) may also be reported. A mortality rate can be expressed as the percentage of the population that has died from a disease or as the number of deaths per 100,000 persons (or other suitable standard number).
Exercise \(\PageIndex{1}\)
- Explain the difference between incidence and prevalence.
- Describe how morbidity and mortality rates are expressed.
Patterns of Incidence
Diseases that are seen only occasionally, and usually without geographic concentration, are called sporadic diseases. Examples of sporadic diseases include tetanus, rabies, and plague. In the United States, Clostridium tetani, the bacterium that causes tetanus, is ubiquitous in the soil environment, but incidences of infection occur only rarely and in scattered locations because most individuals are vaccinated, clean wounds appropriately, or are only rarely in a situation that would cause infection.3 Likewise in the United States there are a few scattered cases of plague each year, usually contracted from rodents in rural areas in the western states.4
Diseases that are constantly present (often at a low level) in a population within a particular geographic region are called endemic diseases. For example, malaria is endemic to some regions of Brazil, but is not endemic to the United States.
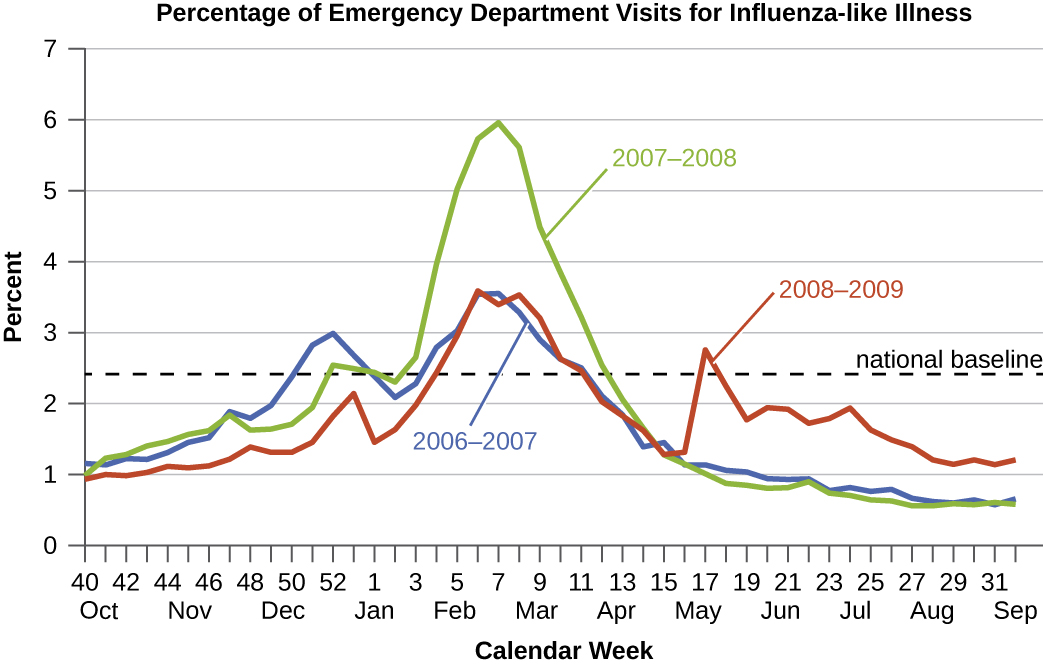
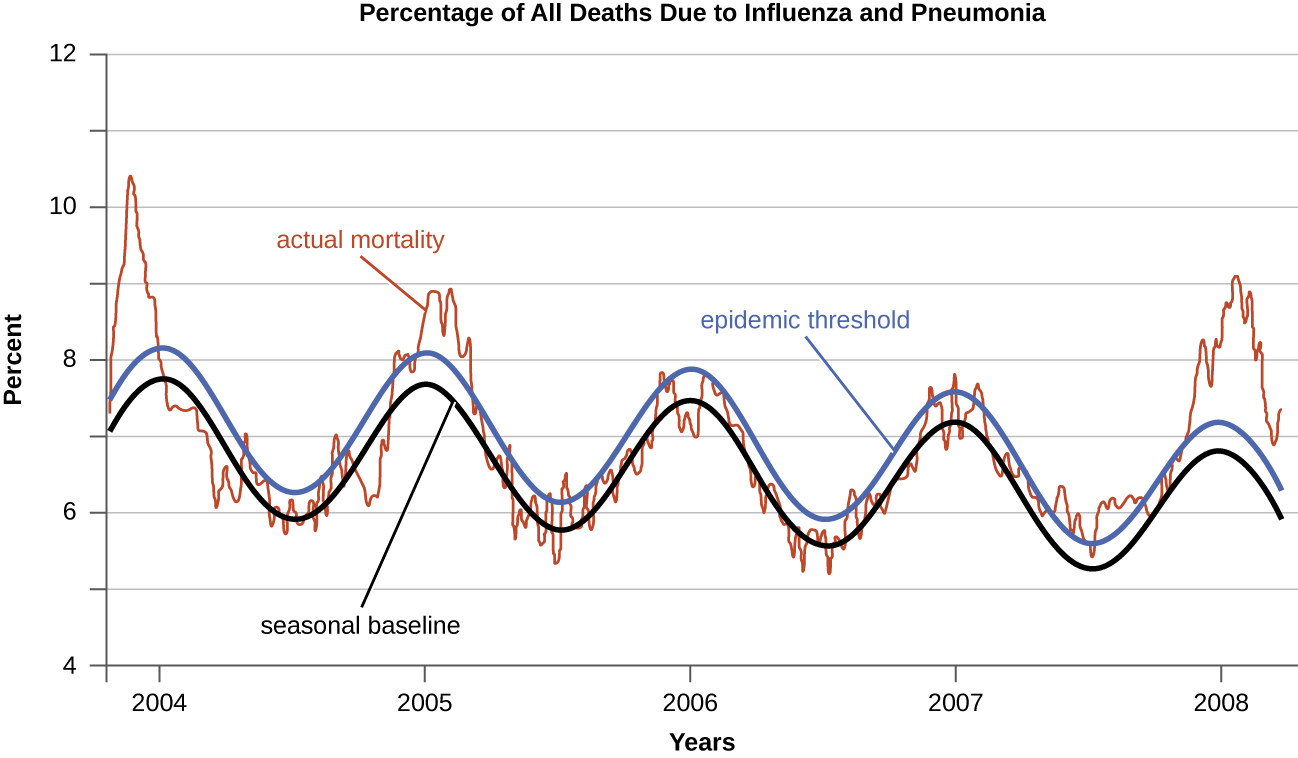
Diseases for which a larger than expected number of cases occurs in a short time within a geographic region are called epidemic diseases. Influenza is a good example of a commonly epidemic disease. Incidence patterns of influenza tend to rise each winter in the northern hemisphere. These seasonal increases are expected, so it would not be accurate to say that influenza is epidemic every winter; however, some winters have an usually large number of seasonal influenza cases in particular regions, and such situations would qualify as epidemics (Figure \(\PageIndex{2}\) and Figure \(\PageIndex{3}\)).
An epidemic disease signals the breakdown of an equilibrium in disease frequency, often resulting from some change in environmental conditions or in the population. In the case of influenza, the disruption can be due to antigenic shift or drift, which allows influenza virus strains to circumvent the acquired immunity of their human hosts.
An epidemic that occurs on a worldwide scale is called a pandemic disease. For example, HIV/AIDS is a pandemic disease and novel influenza virus strains often become pandemic.
Exercise \(\PageIndex{2}\)
- Explain the difference between sporadic and endemic disease.
- Explain the difference between endemic and epidemic disease.
Etiology
When studying an epidemic, an epidemiologist’s first task is to determinate the cause of the disease, called the etiologic agent or causative agent. Connecting a disease to a specific pathogen can be challenging because of the extra effort typically required to demonstrate direct causation as opposed to a simple association. It is not enough to observe an association between a disease and a suspected pathogen; controlled experiments are needed to eliminate other possible causes. In addition, pathogens are typically difficult to detect when there is no immediate clue as to what is causing the outbreak. Signs and symptoms of disease are also commonly nonspecific, meaning that many different agents can give rise to the same set of signs and symptoms. This complicates diagnosis even when a causative agent is familiar to scientists.
Robert Koch was the first scientist to specifically demonstrate the causative agent of a disease (anthrax) in the late 1800s. Koch developed four criteria, now known as Koch’s postulates, which had to be met in order to positively link a disease with a pathogenic microbe. Without Koch’s postulates, the Golden Age of Microbiology would not have occurred. Between 1876 and 1905, many common diseases were linked with their etiologic agents, including cholera, diphtheria, gonorrhea, meningitis, plague, syphilis, tetanus, and tuberculosis. Today, we use the molecular Koch’s postulates, a variation of Koch’s original postulates that can be used to establish a link between the disease state and virulence traits unique to a pathogenic strain of a microbe.
Koch’s Postulates
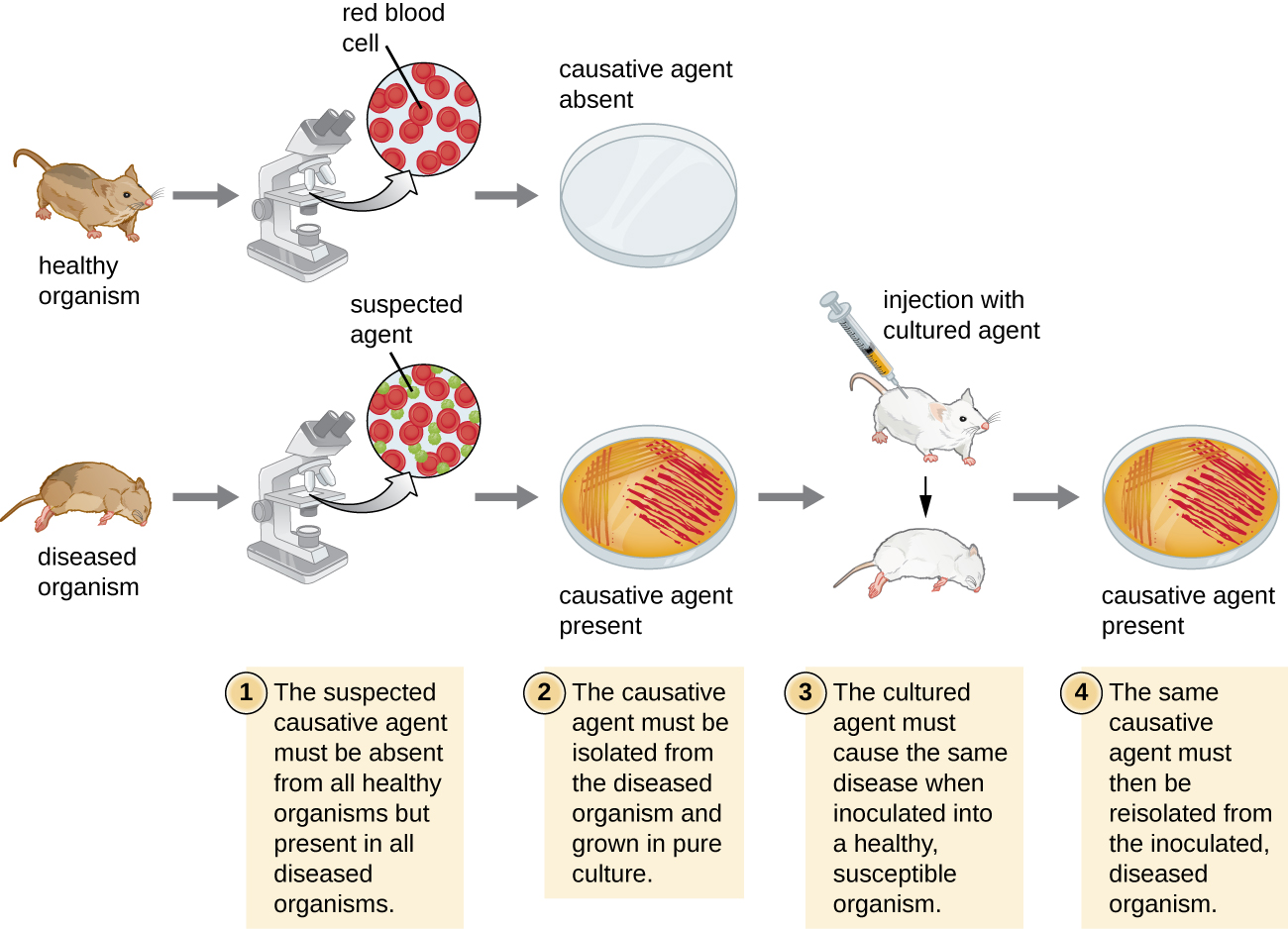
In 1884, Koch published four postulates that summarized his method for determining whether a particular microorganism was the cause of a particular disease. Each of Koch’s postulates represents a criterion that must be met before a disease can be positively linked with a pathogen. In order to determine whether the criteria are met, tests are performed on laboratory animals and cultures from healthy and diseased animals are compared (Figure \(\PageIndex{4}\)).
Koch’s Postulates
- The suspected pathogen must be found in every case of disease and not be found in healthy individuals.
- The suspected pathogen can be isolated and grown in pure culture.
- A healthy test subject infected with the suspected pathogen must develop the same signs and symptoms of disease as seen in postulate
- The pathogen must be re-isolated from the new host and must be identical to the pathogen from postulate 2.
In many ways, Koch’s postulates are still central to our current understanding of the causes of disease. However, advances in microbiology have revealed some important limitations in Koch’s criteria. Koch made several assumptions that we now know are untrue in many cases. The first relates to postulate 1, which assumes that pathogens are only found in diseased, not healthy, individuals. This is not true for many pathogens. For example, H. pylori, described earlier in this chapter as a pathogen causing chronic gastritis, is also part of the normal microbiota of the stomach in many healthy humans who never develop gastritis. It is estimated that upwards of 50% of the human population acquires H. pylori early in life, with most maintaining it as part of the normal microbiota for the rest of their life without ever developing disease.
Koch’s second faulty assumption was that all healthy test subjects are equally susceptible to disease. We now know that individuals are not equally susceptible to disease. Individuals are unique in terms of their microbiota and the state of their immune system at any given time. The makeup of the resident microbiota can influence an individual’s susceptibility to an infection. Members of the normal microbiota play an important role in immunity by inhibiting the growth of transient pathogens. In some cases, the microbiota may prevent a pathogen from establishing an infection; in others, it may not prevent an infection altogether but may influence the severity or type of signs and symptoms. As a result, two individuals with the same disease may not always present with the same signs and symptoms. In addition, some individuals have stronger immune systems than others. Individuals with immune systems weakened by age or an unrelated illness are much more susceptible to certain infections than individuals with strong immune systems.
Koch also assumed that all pathogens are microorganisms that can be grown in pure culture (postulate 2) and that animals could serve as reliable models for human disease. However, we now know that not all pathogens can be grown in pure culture, and many human diseases cannot be reliably replicated in animal hosts. Viruses and certain bacteria, including Rickettsia and Chlamydia, are obligate intracellular pathogens that can grow only when inside a host cell. If a microbe cannot be cultured, a researcher cannot move past postulate 2. Likewise, without a suitable nonhuman host, a researcher cannot evaluate postulate 2 without deliberately infecting humans, which presents obvious ethical concerns. AIDS is an example of such a disease because the human immunodeficiency virus (HIV) only causes disease in humans.
Exercise \(\PageIndex{3}\)
Briefly summarize the limitations of Koch’s postulates.
Molecular Koch’s Postulates
In 1988, Stanley Falkow (1934–) proposed a revised form of Koch’s postulates known as molecular Koch’s postulates. These are listed in the left column of Table \(\PageIndex{1}\). The premise for molecular Koch’s postulates is not in the ability to isolate a particular pathogen but rather to identify a gene that may cause the organism to be pathogenic.
Falkow’s modifications to Koch’s original postulates explain not only infections caused by intracellular pathogens but also the existence of pathogenic strains of organisms that are usually nonpathogenic. For example, the predominant form of the bacterium Escherichia coli is a member of the normal microbiota of the human intestine and is generally considered harmless. However, there are pathogenic strains of E. coli such as enterotoxigenic E. coli (ETEC) and enterohemorrhagic E. coli (O157:H7) (EHEC). We now know ETEC and EHEC exist because of the acquisition of new genes by the once-harmless E. coli, which, in the form of these pathogenic strains, is now capable of producing toxins and causing illness. The pathogenic forms resulted from minor genetic changes. The right-side column of Table \(\PageIndex{1}\) illustrates how molecular Koch’s postulates can be applied to identify EHEC as a pathogenic bacterium.
| Molecular Koch’s Postulates | Application to EHEC |
|---|---|
| (1) The phenotype (sign or symptom of disease) should be associated only with pathogenic strains of a species. | EHEC causes intestinal inflammation and diarrhea, whereas nonpathogenic strains of E. coli do not. |
| (2) Inactivation of the suspected gene(s) associated with pathogenicity should result in a measurable loss of pathogenicity. | One of the genes in EHEC encodes for Shiga toxin, a bacterial toxin (poison) that inhibits protein synthesis. Inactivating this gene reduces the bacteria’s ability to cause disease. |
| (3) Reversion of the inactive gene should restore the disease phenotype. | By adding the gene that encodes the toxin back into the genome (e.g., with a phage or plasmid), EHEC’s ability to cause disease is restored. |
As with Koch’s original postulates, the molecular Koch’s postulates have limitations. For example, genetic manipulation of some pathogens is not possible using current methods of molecular genetics. In a similar vein, some diseases do not have suitable animal models, which limits the utility of both the original and molecular postulates.
Exercise \(\PageIndex{4}\)
- Explain the differences between Koch’s original postulates and the molecular Koch’s postulates.
- List some challenges to determining the causative agent of a disease outbreak.
The Role of Public Health Organizations
The main national public health agency in the United States is the Centers for Disease Control and Prevention (CDC), an agency of the Department of Health and Human Services. The CDC is charged with protecting the public from disease and injury. One way that the CDC carries out this mission is by overseeing the National Notifiable Disease Surveillance System (NNDSS) in cooperation with regional, state, and territorial public health departments. The NNDSS monitors diseases considered to be of public health importance on a national scale. Such diseases are called notifiable diseases or reportable diseases because all cases must be reported to the CDC. A physician treating a patient with a notifiable disease is legally required to submit a report on the case. Notifiable diseases include HIV infection, measles, West Nile virus infections, and many others. Some states have their own lists of notifiable diseases that include diseases beyond those on the CDC’s list.
Notifiable diseases are tracked by epidemiological studies and the data is used to inform health-care providers and the public about possible risks. The CDC publishes the Morbidity and Mortality Weekly Report (MMWR), which provides physicians and health-care workers with updates on public health issues and the latest data pertaining to notifiable diseases. Table \(\PageIndex{2}\) is an example of the kind of data contained in the MMWR.
Table \(\PageIndex{2}\): Incidence of Four Notifiable Diseases in the United States, Week Ending January 2, 2016
| Disease | Current Week (Jan 2, 2016) | Median of Previous 52 Weeks | Maximum of Previous 52 Weeks | Cumulative Cases 2015 |
|---|---|---|---|---|
| Campylobacteriosis | 406 | 869 | 1,385 | 46,618 |
| Chlamydia trachomatis infection | 11,024 | 28,562 | 31,089 | 1,425,303 |
| Giardiasis | 115 | 230 | 335 | 11,870 |
| Gonorrhea | 3,207 | 7,155 | 8,283 | 369,926 |
The current Morbidity and Mortality Weekly Report is available online.
Exercise \(\PageIndex{5}\)
Describe how health agencies obtain data about the incidence of diseases of public health importance.
The World Health Organization (WHO)
A large number of international programs and agencies are involved in efforts to promote global public health. Among their goals are developing infrastructure in health care, public sanitation, and public health capacity; monitoring infectious disease occurrences around the world; coordinating communications between national public health agencies in various countries; and coordinating international responses to major health crises. In large part, these international efforts are necessary because disease-causing microorganisms know no national boundaries.
International public health issues are coordinated by the World Health Organization (WHO), an agency of the United Nations. Of its roughly $4 billion budget for 2015–165, about $1 billion was funded by member states and the remaining $3 billion by voluntary contributions. In addition to monitoring and reporting on infectious disease, WHO also develops and implements strategies for their control and prevention. WHO has had a number of successful international public health campaigns. For example, its vaccination program against smallpox, begun in the mid-1960s, resulted in the global eradication of the disease by 1980. WHO continues to be involved in infectious disease control, primarily in the developing world, with programs targeting malaria, HIV/AIDS, and tuberculosis, among others. It also runs programs to reduce illness and mortality that occur as a result of violence, accidents, lifestyle-associated illnesses such as diabetes, and poor health-care infrastructure.
WHO maintains a global alert and response system that coordinates information from member nations. In the event of a public health emergency or epidemic, it provides logistical support and coordinates international response to the emergency. The United States contributes to this effort through the CDC. The CDC carries out international monitoring and public health efforts, mainly in the service of protecting US public health in an increasingly connected world. Similarly, the European Union maintains a Health Security Committee that monitors disease outbreaks within its member countries and internationally, coordinating with WHO.
Exercise \(\PageIndex{6}\)
Name the organizations that participate in international public health monitoring.
Emerging and Reemerging Infectious Diseases
Both WHO and some national public health agencies such as the CDC monitor and prepare for emerging infectious diseases. An emerging infectious disease is either new to the human population or has shown an increase in prevalence in the previous twenty years. Whether the disease is new or conditions have changed to cause an increase in frequency, its status as emerging implies the need to apply resources to understand and control its growing impact.
Emerging diseases may change their frequency gradually over time, or they may experience sudden epidemic growth. The importance of vigilance was made clear during the Ebola hemorrhagic fever epidemic in western Africa through 2014–2015. Although health experts had been aware of the Ebola virus since the 1970s, an outbreak on such a large scale had never happened before (Figure \(\PageIndex{5}\)). Previous human epidemics had been small, isolated, and contained. Indeed, the gorilla and chimpanzee populations of western Africa had suffered far worse from Ebola than the human population. The pattern of small isolated human epidemics changed in 2014. Its high transmission rate, coupled with cultural practices for treatment of the dead and perhaps its emergence in an urban setting, caused the disease to spread rapidly, and thousands of people died. The international public health community responded with a large emergency effort to treat patients and contain the epidemic.
Emerging diseases are found in all countries, both developed and developing (Table \(\PageIndex{3}\)). Some nations are better equipped to deal with them. National and international public health agencies watch for epidemics like the Ebola outbreak in developing countries because those countries rarely have the health-care infrastructure and expertise to deal with large outbreaks effectively. Even with the support of international agencies, the systems in western Africa struggled to identify and care for the sick and control spread. In addition to the altruistic goal of saving lives and assisting nations lacking in resources, the global nature of transportation means that an outbreak anywhere can spread quickly to every corner of the planet. Managing an epidemic in one location—its source—is far easier than fighting it on many fronts.
Ebola is not the only disease that needs to be monitored in the global environment. In 2015, WHO set priorities on several emerging diseases that had a high probability of causing epidemics and that were poorly understood (and thus urgently required research and development efforts).
A reemerging infectious disease is a disease that is increasing in frequency after a previous period of decline. Its reemergence may be a result of changing conditions or old prevention regimes that are no longer working. Examples of such diseases are drug-resistant forms of tuberculosis, bacterial pneumonia, and malaria. Drug-resistant strains of the bacteria causing gonorrhea and syphilis are also becoming more widespread, raising concerns of untreatable infections.
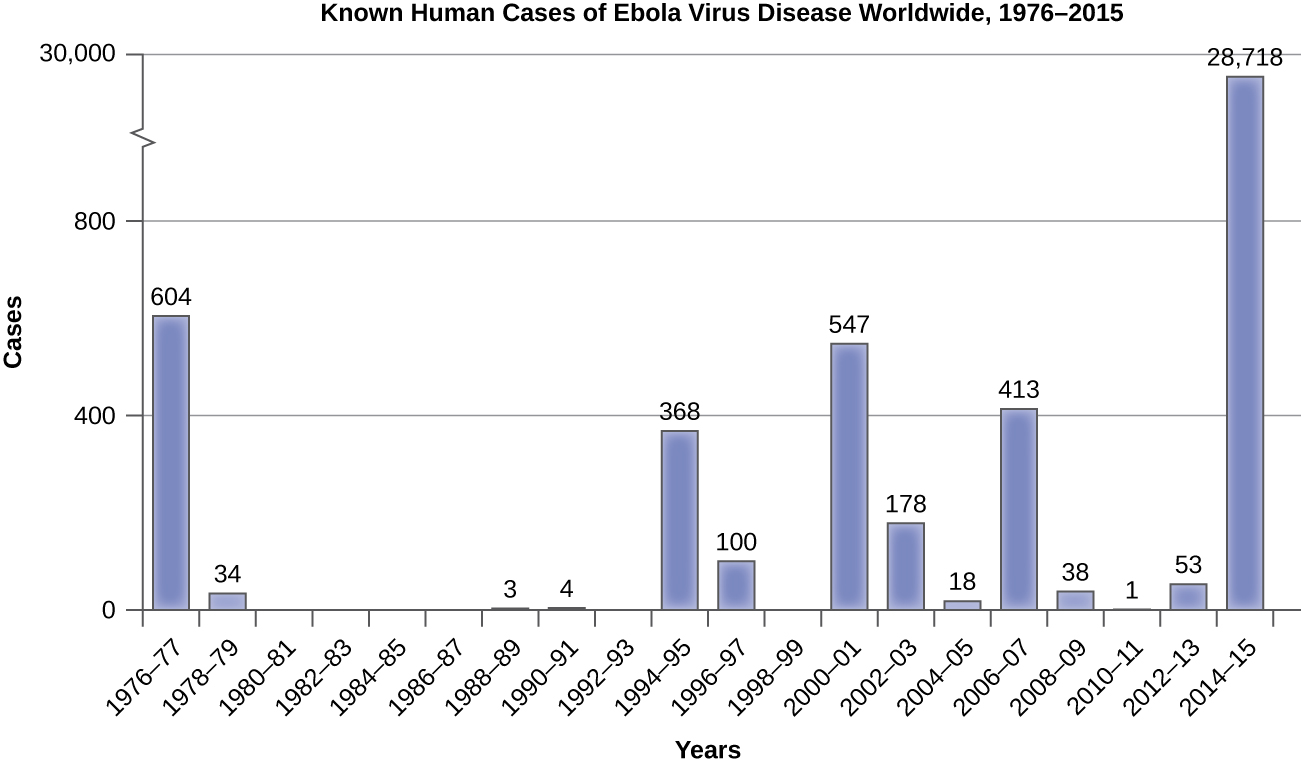
Table \(\PageIndex{3}\): Some Emerging and Reemerging Infectious Diseases
| Disease | Pathogen | Year Discovered | Affected Regions | Transmission |
|---|---|---|---|---|
| AIDS | HIV | 1981 | Worldwide | Contact with infected body fluids |
| Chikungunya fever | Chikungunya virus | 1952 | Africa, Asia, India; spreading to Europe and the Americas | Mosquito-borne |
| Ebola virus disease | Ebola virus | 1976 | Central and Western Africa | Contact with infected body fluids |
| H1N1 Influenza (swine flu) | H1N1 virus | 2009 | Worldwide | Droplet transmission |
| Lyme disease | Borrelia burgdorferi bacterium | 1981 | Northern hemisphere | From mammal reservoirs to humans by tick vectors |
| West Nile virus disease | West Nile virus | 1937 | Africa, Australia, Canada to Venezuela, Europe, Middle East, Western Asia | Mosquito-borne |
Exercise \(\PageIndex{7}\)
- Explain why it is important to monitor emerging infectious diseases.
- Explain how a bacterial disease could reemerge, even if it had previously been successfully treated and controlled.
SARS Outbreak and Identification
On November 16, 2002, the first case of a SARS outbreak was reported in Guangdong Province, China. The patient exhibited influenza-like symptoms such as fever, cough, myalgia, sore throat, and shortness of breath. As the number of cases grew, the Chinese government was reluctant to openly communicate information about the epidemic with the World Health Organization (WHO) and the international community. The slow reaction of Chinese public health officials to this new disease contributed to the spread of the epidemic within and later outside China. In April 2003, the Chinese government finally responded with a huge public health effort involving quarantines, medical checkpoints, and massive cleaning projects. Over 18,000 people were quarantined in Beijing alone. Large funding initiatives were created to improve health-care facilities, and dedicated outbreak teams were created to coordinate the response. By August 16, 2003, the last SARS patients were released from a hospital in Beijing nine months after the first case was reported in China.
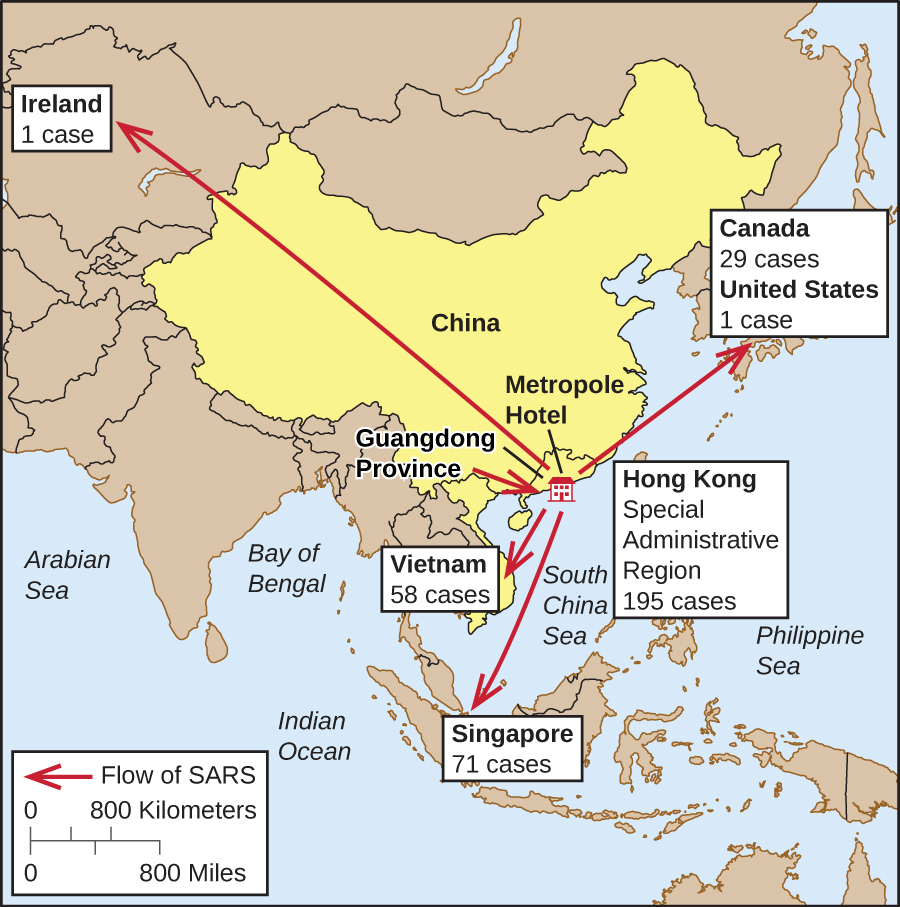
In the meantime, SARS spread to other countries on its way to becoming a global pandemic. Though the infectious agent had yet to be identified, it was thought to be an influenza virus. The disease was named SARS, an acronym for severe acute respiratory syndrome, until the etiologic agent could be identified. Travel restrictions to Southeast Asia were enforced by many countries. By the end of the outbreak, there were 8,098 cases and 774 deaths worldwide. China and Hong Kong were hit hardest by the epidemic, but Taiwan, Singapore, and Toronto, Canada, also saw significant numbers of cases (Figure \(\PageIndex{6}\)).
Fortunately, timely public health responses in many countries effectively suppressed the outbreak and led to its eventual containment. For example, the disease was introduced to Canada in February 2003 by an infected traveler from Hong Kong, who died shortly after being hospitalized. By the end of March, hospital isolation and home quarantine procedures were in place in the Toronto area, stringent anti-infection protocols were introduced in hospitals, and the media were actively reporting on the disease. Public health officials tracked down contacts of infected individuals and quarantined them. A total of 25,000 individuals were quarantined in the city. Thanks to the vigorous response of the Canadian public health community, SARS was brought under control in Toronto by June, a mere four months after it was introduced.
In 2003, WHO established a collaborative effort to identify the causative agent of SARS, which has now been identified as a coronavirus that was associated with horseshoe bats. The genome of the SARS virus was sequenced and published by researchers at the CDC and in Canada in May 2003, and in the same month researchers in the Netherlands confirmed the etiology of the disease by fulfilling Koch’s postulates for the SARS coronavirus. The last known case of SARS worldwide was reported in 2004.
This database of reports chronicles outbreaks of infectious disease around the world. It was on this system that the first information about the SARS outbreak in China emerged.
The CDC publishes Emerging Infectious Diseases, a monthly journal available online.
Key Concepts and Summary
- Epidemiology is the science underlying public health.
- Morbidity means being in a state of illness, whereas mortality refers to death; both morbidity rates and mortality rates are of interest to epidemiologists.
- Incidence is the number of new cases (morbidity or mortality), usually expressed as a proportion, during a specified time period; prevalence is the total number affected in the population, again usually expressed as a proportion.
- Sporadic diseases only occur rarely and largely without a geographic focus. Endemic diseases occur at a constant (and often low) level within a population. Epidemic diseases and pandemic diseases occur when an outbreak occurs on a significantly larger than expected level, either locally or globally, respectively.
- Koch’s postulates specify the procedure for confirming a particular pathogen as the etiologic agent of a particular disease. Koch’s postulates have limitations in application if the microbe cannot be isolated and cultured or if there is no animal host for the microbe. In this case, molecular Koch’s postulates would be utilized.
- In the United States, the Centers for Disease Control and Prevention monitors notifiable diseases and publishes weekly updates in the Morbidity and Mortality Weekly Report.
- The World Health Organization (WHO) is an agency of the United Nations that collects and analyzes data on disease occurrence from member nations. WHO also coordinates public health programs and responses to international health emergencies.
- Emerging diseases are those that are new to human populations or that have been increasing in the past two decades. Reemerging diseases are those that are making a resurgence in susceptible populations after previously having been controlled in some geographic areas.
Footnotes
- H. Irene Hall, Qian An, Tian Tang, Ruiguang Song, Mi Chen, Timothy Green, and Jian Kang. “Prevalence of Diagnosed and Undiagnosed HIV Infection—United States, 2008–2012.” Morbidity and Mortality Weekly Report 64, no. 24 (2015): 657–662.
- Centers for Disease Control and Prevention. “Diagnoses of HIV Infection in the United States and Dependent Areas, 2014.” HIV Surveillance Report 26 (2015).
- Centers for Disease Control and Prevention. “Tetanus Surveillance—United States, 2001–2008.” Morbidity and Mortality Weekly Report 60, no. 12 (2011): 365.
- Centers for Disease Control and Prevention. “Plague in the United States.” 2015. http://www.cdc.gov/plague/maps. Accessed June 1, 2016.
- World Health Organization. “Programme Budget 2014–2015.” www.who.int/about/finances-ac...lity/budget/en.
Contributors and Attributions
Nina Parker, (Shenandoah University), Mark Schneegurt (Wichita State University), Anh-Hue Thi Tu (Georgia Southwestern State University), Philip Lister (Central New Mexico Community College), and Brian M. Forster (Saint Joseph’s University) with many contributing authors. Original content via Openstax (CC BY 4.0; Access for free at https://openstax.org/books/microbiology/pages/1-introduction)


